Page 197 - Haematologica April 2020
P. 197
Daratumumab in B-NHL
AB
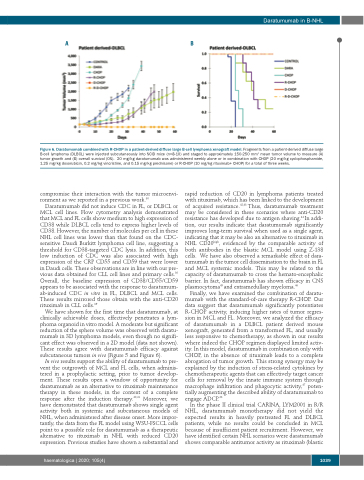
Figure 6. Daratumumab combined with R-CHOP in a patient-derived diffuse large B-cell lymphoma xenograft model. Fragments from a patient-derived diffuse large B-cell lymphoma (DLBCL) were injected subcutaneously into SCID mice (n=8-10) and staged to approximately 150-250 mm3 mean tumor volume to measure (A) tumor growth and (B) overall survival (OS). 20 mg/kg daratumumab was administered weekly alone or in combination with CHOP (20 mg/kg cyclophosphamide, 1.25 mg/kg doxorubicin, 0.2 mg/kg vincristine, and 0.15 mg/kg prednisone) or R-CHOP (10 mg/kg rituximab+ CHOP) for a total of three weeks.
compromise their interaction with the tumor microenvi- ronment as we reported in a previous work.30
Daratumumab did not induce CDC in FL, or DLBCL or MCL cell lines. Flow cytometry analysis demonstrated that MCL and FL cells show medium to high expression of CD38 while DLBCL cells tend to express higher levels of CD38. However, the number of molecules per cell in these NHL cell lines was lower than that found on the CDC- sensitive Daudi Burkitt lymphoma cell line, suggesting a threshold for CD38-targeted CDC lysis. In addition, this low induction of CDC was also associated with high expression of the CRP CD55 and CD59 that were lower in Daudi cells. These observations are in line with our pre- vious data obtained for CLL cell lines and primary cells.30 Overall, the baseline expression of CD38/CD55/CD59 appears to be associated with the response to daratumum- ab-induced CDC in vitro in FL, DLBCL and MCL cells. These results mirrored those obtain with the anti-CD20 rituximab in CLL cells.39
We have shown for the first time that daratumumab, at clinically achievable doses, effectively penetrates a lym- phoma organoid in vitro model. A moderate but significant reduction of the sphere volume was observed with daratu- mumab in 3D lymphoma models, even though no signifi- cant effect was observed in a 2D model (data not shown). These results agree with daratumumab efficacy against subcutaneous tumors in vivo (Figure 5 and Figure 6).
In vivo results support the ability of daratumumab to pre- vent the outgrowth of MCL and FL cells, when adminis- tered in a prophylactic setting, prior to tumor develop- ment. These results open a window of opportunity for daratumumab as an alternative to rituximab maintenance therapy in these models, in the context of a complete response after the induction therapy.40,41 Moreover, we have demonstrated that daratumumab shows single agent activity both in systemic and subcutaneous models of NHL, when administered after disease onset. More impor- tantly, the data from the FL model using WSU-FSCCL cells point to a possible role for daratumumab as a therapeutic alternative to rituximab in NHL with reduced CD20 expression. Previous studies have shown a substantial and
rapid reduction of CD20 in lymphoma patients treated with rituximab, which has been linked to the development of acquired resistance.42,43 Thus, daratumumab treatment may be considered in these scenarios where anti-CD20 resistance has developed due to antigen shaving.44 In addi- tion, our results indicate that daratumumab significantly improves long-term survival when used as a single agent, indicating that it may be also an alternative to rituximab in NHL CD20high, evidenced by the comparable activity of both antibodies in the blastic MCL model using Z-138 cells. We have also observed a remarkable effect of dara- tumumab in the tumor cell dissemination to the brain in FL and MCL systemic models. This may be related to the capacity of daratumumab to cross the hemato-encephalic barrier. In fact, daratumumab has shown efficacy in CNS plasmocytoma45 and extramedullary myeloma.46
Finally, we have examined the combination of daratu- mumab with the standard-of-care therapy R-CHOP. Our data suggest that daratumumab significantly potentiates R-CHOP activity, inducing higher rates of tumor regres- sion in MCL and FL. Moreover, we analyzed the efficacy of daratumumab in a DLBCL patient derived mouse xenograft, generated from a transformed FL, and usually less responsive to chemotherapy, as shown in our results where indeed the CHOP regimen displayed limited activ- ity. In this model, daratumumab in combination only with CHOP, in the absence of rituximab leads to a complete abrogation of tumor growth. This strong synergy may be explained by the induction of stress-related cytokines by chemotherapeutic agents that can effectively target cancer cells for removal by the innate immune system through macrophage infiltration and phagocytic activity,47 poten- tially augmenting the described ability of daratumumab to engage ADCP.26
In the phase II clinical trial CARINA, LYM2001 in R/R NHL, daratumumab monotherapy did not yield the expected results in heavily pretreated FL and DLBCL patients, while no results could be concluded in MCL because of insufficient patient recruitment. However, we have identified certain NHL scenarios were daratumumab shows comparable antitumor activity as rituximab (blastic
haematologica | 2020; 105(4)
1039