Page 196 - Haematologica April 2020
P. 196
A. Vidal-Crespo et al.
AB
CD
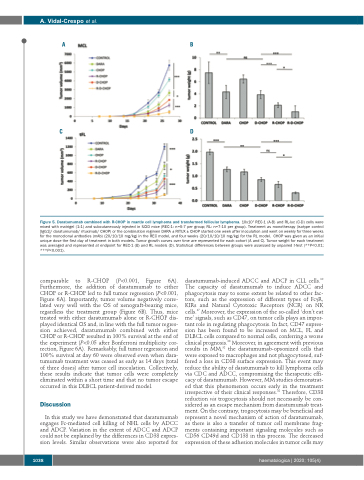
Figure 5. Daratumumab combined with R-CHOP in mantle cell lymphoma and transformed follicular lymphoma. 10x106 REC-1 (A-B) and RL-luc (C-D) cells were mixed with matrigel (1:1) and subcutaneously injected in SCID mice (REC-1: n=5-7 per group; RL: n=7-10 per group). Treatment as monotherapy (isotype control [IgG1]/ daratumumab/ rituximab/ CHOP) or the combination regimen DARA ± RITUX ± CHOP started one week after inoculation and went on weekly for three weeks for the monoclonal antbodies (mAb) (20/10/10 mg/kg) in the REC model, and four weeks (20/10/10/10 mg/kg) for the RL model. CHOP was given as an initial unique dose the first day of treatment in both models. Tumor growth curves over time are represented for each cohort (A and C). Tumor weight for each treatment was averaged and represented at endpoint for REC-1 (B) and RL models (D). Statistical differences between groups were assessed by unpaired t-test (**P<0.01; ***P<0.001).
comparable to R-CHOP (P<0.001, Figure 6A). Furthermore, the addition of daratumumab to either CHOP or R-CHOP led to full tumor regression (P<0.001, Figure 6A). Importantly, tumor volume negatively corre- lated very well with the OS of xenograft-bearing mice, regardless the treatment group (Figure 6B). Thus, mice treated with either daratumumab alone or R-CHOP dis- played identical OS and, in line with the full tumor regres- sion achieved, daratumumab combined with either CHOP or R-CHOP resulted in 100% survival at the end of the experiment (P<0.05 after Bonferroni multiplicity cor- rection, Figure 6A). Remarkably, full tumor regression and 100% survival at day 60 were observed even when dara- tumumab treatment was ceased as early as 14 days (total of three doses) after tumor cell inoculation. Collectively, these results indicate that tumor cells were completely eliminated within a short time and that no tumor escape occurred in this DLBCL patient-derived model.
Discussion
In this study we have demonstrated that daratumumab engages Fc-mediated cell killing of NHL cells by ADCC and ADCP. Variation in the extent of ADCC and ADCP could not be explained by the differences in CD38 expres- sion levels. Similar observations were also reported for
daratumumab-induced ADCC and ADCP in CLL cells.30 The capacity of daratumumab to induce ADCC and phagocytosis may to some extent be related to other fac- tors, such as the expression of different types of FcγR, KIRs and Natural Cytotoxic Receptors (NCR) on NK cells.37 Moreover, the expression of the so-called ‘don’t eat me’ signals, such as CD47, on tumor cells plays an impor- tant role in regulating phagocytosis. In fact, CD47 expres- sion has been found to be increased on MCL, FL and DLBCL cells compared to normal cells, conferring a worse clinical prognosis.38 Moreover, in agreement with previous results in MM,32 the daratumumab-opsonized cells that were exposed to macrophages and not phagocytosed, suf- fered a loss in CD38 surface expression. This event may reduce the ability of daratumumab to kill lymphoma cells via CDC and ADCC, compromising the therapeutic effi- cacy of daratumumab. However, MM studies demonstrat- ed that this phenomenon occurs early in the treatment irrespective of their clinical responses.32 Therefore, CD38 reduction via trogocytosis should not necessarily be con- sidered as an escape mechanism from daratumumab treat- ment. On the contrary, trogocytosis may be beneficial and represent a novel mechanism of action of daratumumab, as there is also a transfer of tumor cell membrane frag- ments containing important signaling molecules such as CD56 CD49d and CD138 in this process. The decreased expression of these adhesion molecules in tumor cells may
1038
haematologica | 2020; 105(4)