Page 193 - Haematologica April 2020
P. 193
Daratumumab in B-NHL
ABC
DEF
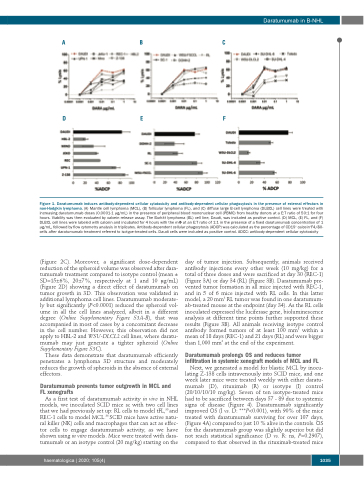
Figure 1. Daratumumab induces antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis in the presence of external effectors in non-Hodgkin lymphoma. (A) Mantle cell lymphoma (MCL), (B) follicular lymphoma (FL), and (C) diffuse large B-cell lymphoma (DLBCL) cell lines were treated with increasing daratumumab doses (0.0001-1 μg/mL) in the presence of peripheral blood mononuclear cell (PBMC) from healthy donors at a E:T ratio of 50:1 for four hours. Viability was then evaluated by calcein release assay. The Burkitt lymphoma (BL) cell line, Daudi, was included as positive control. (D) MCL, (E) FL, and (F) DLBCL cell lines were labeled with calcein and incubated for 4 hours with the mΦ at an E:T ratio of 1:1 in the presence of a fixed daratumumab concentration of 1 μg/mL, followed by flow cytometry analysis in triplicates. Antibody-dependent cellular phagocytosis (ADCP) was calculated as the percentage of CD19+ calcein+F4/80- cells after daratumumab treatment referred to isotype-treated cells. Daudi cells were included as positive control. ADCC: antibody-dependent cellular cytotoxicity
(Figure 2C). Moreover, a significant dose-dependent reduction of the spheroid volume was observed after dara- tumumab treatment compared to isotype control (mean ± SD=15±6%, 20±7%, respectively at 1 and 10 μg/mL) (Figure 2D) showing a direct effect of daratumumab on tumor growth in 3D. This observation was validated in additional lymphoma cell lines. Daratumumab moderate- ly but significantly (P<0.0001) reduced the spheroid vol- ume in all the cell lines analyzed, albeit in a different degree (Online Supplementary Figure S3A-B), that was accompanied in most of cases by a concomitant decrease in the cell number. However, this observation did not apply to HBL-2 and WSU-DLCL2 cell lines, where daratu- mumab may just generate a tighter spheroid (Online Supplementary Figure S3C).
These data demonstrate that daratumumab efficiently penetrates a lymphoma 3D structure and moderately reduces the growth of spheroids in the absence of external effectors.
Daratumumab prevents tumor outgrowth in MCL and FL xenografts
As a first test of daratumumab activity in vivo in NHL models, we inoculated SCID mice sc with two cell lines that we had previously set up: RL cells to model tFL,35 and REC-1 cells to model MCL.36 SCID mice have active natu- ral killer (NK) cells and macrophages that can act as effec- tor cells to engage daratumumab activity, as we have shown using in vitro models. Mice were treated with dara- tumumab or an isotype control (20 mg/kg) starting on the
day of tumor injection. Subsequently, animals received antibody injections every other week (10 mg/kg) for a total of three doses and were sacrificed at day 30 (REC-1) (Figure 3A) or day 34 (RL) (Figure 3B). Daratumumab pre- vented tumor formation in all mice injected with REC-1, and in 5 of 6 mice injected with RL cells. In this latter model, a 20 mm3 RL tumor was found in one daratumum- ab-treated mouse at the endpoint (day 34). As the RL cells inoculated expressed the luciferase gene, bioluminescence analysis at different time points further supported these results (Figure 3B). All animals receiving isotype control antibody formed tumors of at least 100 mm3 within a mean of 18 days (REC-1) and 21 days (RL) and were bigger than 1,000 mm3 at the end of the experiment.
Daratumumab prolongs OS and reduces tumor infiltration in systemic xenograft models of MCL and FL
Next, we generated a model for blastic MCL by inocu- lating Z-138 cells intravenously into SCID mice, and one week later mice were treated weekly with either daratu- mumab (D), rituximab (R) or isotype (I) control (20/10/10/10 mg/kg). Seven of ten isotype-treated mice had to be sacrificed between days 57 - 89 due to systemic signs of disease (Figure 4). Daratumumab significantly improved OS (I vs. D: ***P<0.001), with 90% of the mice treated with daratumumab surviving for over 107 days, (Figure 4A) compared to just 10 % alive in the controls. OS for the daratumumab group was slightly superior but did not reach statistical significance (D vs. R: ns, P=0.2907), compared to that observed in the rituximab-treated mice
haematologica | 2020; 105(4)
1035