Page 192 - Haematologica April 2020
P. 192
A. Vidal-Crespo et al.
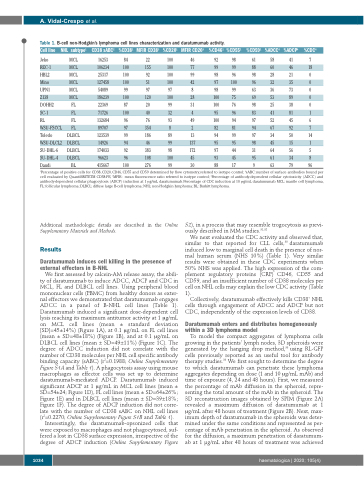
Table 1. B-cell non-Hodgkin’s lymphoma cell lines characterization and daratumumab activity.
Cell line NHL subtype1 CD38 sABC2 %CD381 MFIR CD383 %CD201 MFIR CD203 %CD461 %CD551
Jeko MCL 16253 84 22 100 46 92 98
%CD591 %ADCC4 %ADCP4 %CDC4
61 58 41 7
REC-1 MCL 106234 100 155 100 77 99 99 88 60 HBL2 MCL 25317 100 92 100 99 98 96 98 28 Mino MCL 127458 100 51 100 42 97 100 96 32
UPN1 MCL 54089 99 97 97 8 98 Z138 MCL 186239 100 120 100 28 100
46 18 21 0 35 0 73 0 89 0 38 0 81 1 45 6 92 7 50 14 15 1 56 5 34 8
DOHH2 FL 22369 87 20 99 SC-1 FL 71726 100 40 32
31 100 4 95 49 100
99 63 36
75 69 53
76 98 25
96 83 41
94 97 52
81 94 67
99 97 34
95 98 45
44 31 64
45 95 61
17 9 63 79 96
RL FL 132684 WSU-FSCCL FL 89707
Toledo DLBCL 123539 WSU-DLCL2 DLBCL 14926
SU-DHL-6 DLBCL 174033
SU-DHL-4 DLBCL 96623
Daudi BL 415667 100
76 93 354 8 186 89
46 99 383 98
96 97 99 94 92 96
2
13
137
172
82
94
95
97
93
88
108 100 45
276 99 30
1Percentage of positive cells for CD38, CD20, CD46, CD55 and CD59 determined by flow cytometry, referred to isotype control; 2sABC: number of surface antibodies bound per cell evaluated by QuantiBRITETM CD38-PE; 3MFIR: mean fluorescence ratio referred to isotype control; 4Percentage of antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) induction at 1 μg/mL daratumumab. Percentage of CDC induction at 10 μg/mL daratumumab. MCL: mantle cell lymphoma, FL: follicular lymphoma; DLBCL: diffuse large B-cell lymphoma; NHL: non-Hodgkin lymphoma; BL: Burkitt lymphoma.
Additional methodologic details are described in the Online Supplementary Materials and Methods.
Results
Daratumumab induces cell killing in the presence of external effectors in B-NHL
We first assessed by calcein-AM release assay, the abili- ty of daratumumab to induce ADCC, ADCP and CDC in MCL, FL and DLBCL cell lines. Using peripheral blood mononuclear cells (PBMC) from healthy donors as exter- nal effectors we demonstrated that daratumumab engages ADCC in a panel of B-NHL cell lines (Table 1). Daratumumab induced a significant dose-dependent cell lysis reaching its maximum antitumor activity at 1 μg/mL on MCL cell lines (mean ± standard deviation [SD]=45±14%) (Figure 1A), at 0.1 μg/mL on FL cell lines (mean ± SD=48±18%) (Figure 1B), and at 0.1 μg/mL on DLBCL cell lines (mean ± SD=49±11%) (Figure 1C). The degree of ADCC induction did not correlate with the number of CD38 molecules per NHL cell specific antibody binding capacity (sABC) (r2=0.1988; Online Supplementary Figure S1A and Table 1). A phagocytosis assay using mouse macrophages as effector cells was set up to determine daratumumab-mediated ADCP. Daratumumab induced significant ADCP at 1 μg/mL in MCL cell lines (mean ± SD=54±24; Figure 1D), FL cell lines (mean ± SD=64±26%; Figure 1E) and in DLBCL cell lines (mean ± SD=39±18%; Figure 1F). The degree of ADCP induction did not corre- late with the number of CD38 sABC on NHL cell lines (r2=0.2270; Online Supplementary Figure S1B and Table 1).
Interestingly, the daratumumab-opsonized cells that were exposed to macrophages and not phagocytosed, suf- fered a lost in CD38 surface expression, irrespective of the degree of ADCP induction (Online Supplementary Figure
S2), in a process that may resemble trogocytosis as previ- ously described in MM studies.31,32
We next evaluated the CDC activity and observed that, similar to that reported for CLL cells,30 daratumumab induced low to marginal cell death in the presence of nor- mal human serum (NHS 10%) (Table 1). Very similar results were obtained in these CDC experiments when 50% NHS was applied. The high expression of the com- plement regulatory proteins (CRP) CD46, CD55 and CD59, and an insufficient number of CD38 molecules per cell on NHL cells may explain the low CDC activity (Table 1).
Collectively, daratumumab effectively kills CD38+ NHL cells through engagement of ADCC and ADCP but not CDC, independently of the expression levels of CD38.
Daratumumab enters and distributes homogeneously within a 3D lymphoma model
To model the compact aggregates of lymphoma cells growing in the patients’ lymph nodes, 3D spheroids were generated by the hanging drop method,33 using RL-GFP cells previously reported as an useful tool for antibody therapy studies.34 We first sought to determine the degree to which daratumumab can penetrate these lymphoma aggregates depending on dose (1 and 10 μg/mL mAb) and time of exposure (4, 24 and 48 hours). First, we measured the percentage of mAb diffusion in the spheroid, repre- senting the total amount of the mAb in the spheroid. The 3D reconstruction images obtained by SPIM (Figure 2A) revealed a maximum diffusion of daratumumab at 1 μg/mL after 48 hours of treatment (Figure 2B). Next, max- imum depth of daratumumab in the spheroids was deter- mined under the same conditions and represented as per- centage of mAb penetration in the spheroid. As observed for the diffusion, a maximum penetration of daratumum- ab at 1 μg/mL after 48 hours of treatment was achieved
1034
haematologica | 2020; 105(4)