Page 175 - Haematologica April 2020
P. 175
MTX-associated toxicity in DS-ALL after HD-MTX
AB
CD
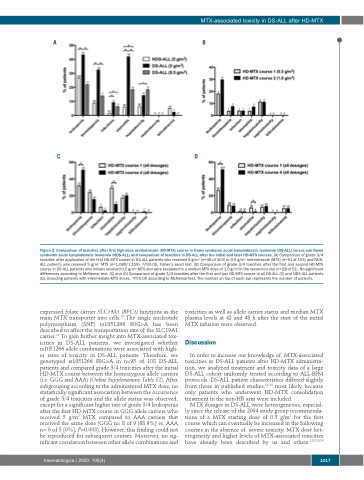
Figure 2. Comparison of toxicities after first high-dose methotrexate (HD-MTX) course in Down syndrome acute lymphoblastic leukemia (DS-ALL) versus non-Down syndrome acute lymphoblastic leukemia (NDS-ALL) and comparison of toxicities in DS-ALL after the initial and later HD-MTX courses. (A) Comparison of grade 3/4 toxicities after application of the first HD-MTX course in DS-ALL patients who received 5 g/m2 (n=45 of 103) or 0.5 g/m2 methotrexate (MTX) (n=51 of 103) and NDS- ALL patients who received 5 g/m2 MTX (n=1,089/1,109). *P≤0.05, Fisher’s exact test. (B) Comparison of grade 3/4 toxicities after the first and second HD-MTX course in DS-ALL patients who initially received 0.5 g/m2 MTX and were escalated to a median MTX dose of 1.0 g/m2 in the second course (n=28 of 51). No significant differences according to McNemar test. (C) and (D) Comparison of grade 3/4 toxicities after the first and last HD-MTX course in all DS-ALL (C) and NDS-ALL patients (D), including patients with intermediate MTX doses. *P≤0.05 according to McNemar-test. The number on top of each bar represents the number of patients.
expressed folate carrier SLC19A1 (RFC1) functions as the main MTX transporter into cells.10 The single nucleotide polymorphism (SNP) rs1051266 80G>A has been described to affect the transportation rate of the SLC19A1 carrier.11 To gain further insight into MTX-associated tox- icities in DS-ALL patients, we investigated whether rs1051266 allele combinations were associated with high- er rates of toxicity in DS-ALL patients. Therefore, we genotyped rs1051266 80G>A in n=95 of 103 DS-ALL patients and compared grade 3/4 toxicities after the initial HD-MTX course between the homozygous allele carriers (i.e. GGG and AAA) (Online Supplementary Table S2). After subgrouping according to the administered MTX dose, no statistically significant association between the occurrence of grade 3/4 toxicities and the allele status was observed, except for a significant higher rate of grade 3/4 leukopenia after the first HD-MTX course in GGG allele carriers who received 5 g/m2 MTX compared to AAA carriers that received the same dose (GGG n= 8 of 9 [88.9%] vs. AAA n= 0 of 5 [0%], P=0.003). However, this finding could not be reproduced for subsequent courses. Moreover, no sig- nificant correlation between other allele combinations and
toxicities as well as allele carrier status and median MTX plasma levels at 42 and 48 h after the start of the initial MTX infusion were observed.
Discussion
In order to increase our knowledge of MTX-associated toxicities in DS-ALL patients after HD-MTX administra- tion, we analyzed treatment and toxicity data of a large DS-ALL cohort uniformly treated according to ALL-BFM protocols. DS-ALL patient characteristics differed slightly from those in published studies,2,6,14 most likely because only patients who underwent HD-MTX consolidation treatment in the non-HR arm were included.
MTX dosages in DS-ALL were heterogeneous, especial- ly since the release of the 2004 study group recommenda- tions of a MTX starting dose of 0.5 g/m2 for the first course which can eventually be increased in the following courses in the absence of severe toxicity. MTX dose het- erogeneity and higher levels of MTX-associated toxicities have already been described by us and others.2,5,8,15,16
haematologica | 2020; 105(4)
1017