Page 173 - Haematologica April 2020
P. 173
MTX-associated toxicity in DS-ALL after HD-MTX
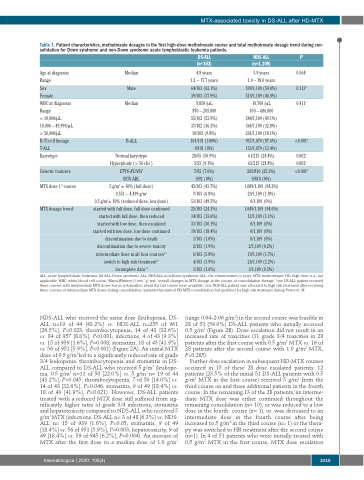
Table 1. Patient characteristics, methotrexate dosages in the first high-dose methotrexate course and total methotrexate dosage trend during con- solidation for Down syndrome and non-Down syndrome acute lymphoblastic leukemia patients.
Age at diagnosis Range
Sex
Female
WBC at diagnosis Range
< 10,000/μL 10,000 – 49,999/μL > 50,000/μL B-/T-cell lineage T-ALL
Karyotype
Genetic features
MTX dose 1st course
MTX dosage trendc
Median
Male
Median
B-ALL
Normal karyotype Hyperploidy (> 50 chr.) ETV6-RUNX1
BCR-ABL
5 g/m2 ± 10% (full dose)
0.551 – 4.499 g/m2
0.5 g/m2± 10% (reduced dose, low dose) started with full dose, full dose continued started with full dose, then reduced started with low dose, then escalated started with low dose, low dose continued discontinuation due to death discontinuation due to severe toxicity intermediate dose in all four coursesd,e switch to high risk treatmente,f incomplete datae,d
DS-ALL (n=103)
4.8 years 1.2 – 17.7 years 64/103 (62.1%) 39/103 (37.9%) 9,850 /μL 870 – 205,000 55/102 (53.9%) 37/102 (36.3%) 10/102 (9.8%) 101/101 (100%) 0/101 (0%) 28/55 (50.9%) 5/55 (9.1%) 7/92 (7.6%) 0/92 (0%) 45/103 (43.7%) 7/103 (6.8%) 51/103 (49.5%) 25/103 (24.3%) 14/103 (13.6%) 31/103 (30.1%) 19/103 (18.4%) 1/103 (1.0%) 2/103 (1.9%) 6/103 (5.8%) 4/103 (3.9%) 1/103 (1.0%)
NDS-ALL (n=1,109)
5.9 years
1.0 – 18.0 years 599/1,109 (54.0%) 510/1,109 (46.0%) 10,700 /μL
100 – 686,000 544/1,109 (49.1%) 364/1,109 (32.8%) 201/1,109 (18.1%) 937/1,070 (87.6%) 133/1,070 (12.4%) 61/215 (28.4%) 62/215 (28.8%) 205/810 (25.3%) 0/810 (0%) 1,089/1,109 (98.2%) 20/1,109 (1.8%) 0/1,109 (0%) 1,049/1,109 (94.6%) 12/1,109 (1.1%) 0/1,109 (0%) 0/1,109 (0%) 0/1,109 (0%) 2/1,109 (0.2%) 19/1,109 (1.7%) 24/1,109 (2.2%) 3/1,109 (0.3%)
P
0.564a
0.113b
0.411a
<0.001b
0.002b
0.002b
<0.001b
ALL: acute lymphoblastic leukemia; DS-ALL: Down syndrome ALL; NDS-ALL: non-Down syndrome ALL; chr: chromosomes; y: years; MTX: methotrexate; HD: high dose; n.a.: not applicable; WBC: white blood cell count; aMann-Whitney U test; bχ2 test; coverall changes in MTX dosage in the course of consolidation therapy; done DS-ALL patient received three courses with intermediate MTX doses but no information about the last course were available; eone NDS-ALL patient was allocated to high risk treatment after receiving three courses of intermediate MTX doses during consolidation; fpatients that started HD-MTX consolidation but qualified for high risk treatment during Protocol M.
NDS-ALL who received the same dose (leukopenia, DS- ALL n=19 of 44 [43.2%] vs. NDS-ALL n=255 of 961 [26.5%], P=0.023; thrombocytopenia, 14 of 43 [32.6%] vs. 84 of 957 [8.8%], P<0.001; infections, 4 of 43 [9.3%] vs. 15 of 939 [1.6%], P=0.008; stomatitis, 18 of 43 [41.9%] vs. 56 of 951 [5.9%], P<0.001) (Figure 2A). An initial MTX dose of 0.5 g/m2 led to a significantly reduced rate of grade 3/4 leukopenia, thrombocytopenia and stomatitis in DS- ALL compared to DS-ALL who received 5 g/m2 (leukope- nia, 0.5 g/m2 n=11 of 50 [22.0%] vs. 5 g/m2 n= 19 of 44 [43.2%], P=0.045; thrombocytopenia, 7 of 50 [14.0%] vs. 14 of 43 [32.6%], P=0.046; stomatitis, 9 of 49 [18.4%] vs. 18 of 43 [41.9%], P=0.021). However, DS-ALL patients treated with a reduced MTX dose still suffered from sig- nificantly higher rates of grade 3/4 infections, stomatitis and hepatotoxicity compared to NDS-ALL who received 5 g/m2 MTX (infections, DS-ALL n= 3 of 48 [6.3%] vs. NDS- ALL n= 15 of 939 [1.6%], P=0.05; stomatitis, 9 of 49 [18.4%] vs. 56 of 951 [5.9%], P=0.003; hepatotoxicity, 9 of 49 [18.4%] vs. 59 of 945 [6.2%], P=0.004). An increase of MTX after the first dose to a median dose of 1.0 g/m2
(range 0.94–2.06 g/m2) in the second course was feasible in 28 of 51 (54.9%) DS-ALL patients who initially received 0.5 g/m2 (Figure 2B). Dose escalation did not result in an increased rate of toxicities (11 grade 3/4 toxicities in 28 patients after the first course with 0.5 g/m2 MTX vs. 16 of 28 patients after the second course with 1.0 g/m2 MTX, P=0.285).
Further dose escalation in subsequent HD-MTX courses occurred in 15 of these 28 dose escalated patients: 12 patients (23.5% of the initial 51 DS-ALL patients with 0.5 g/m2 MTX in the first course) received 5 g/m2 from the third course on and three additional patients in the fourth course. In the remaining 13 of the 28 patients, an interme- diate MTX dose was either continued throughout the remaining consolidation (n= 10), or was reduced to a low dose in the fourth course (n= 1), or was decreased to an intermediate dose in the fourth course after being increased to 5 g/m2 in the third course (n= 1) or the thera- py was switched to HR treatment after the second course (n=1). In 4 of 51 patients who were initially treated with 0.5 g/m2 MTX in the first course, MTX dose escalation
haematologica | 2020; 105(4)
1015