Page 177 - Haematologica April 2020
P. 177
MTX-associated toxicity in DS-ALL after HD-MTX
AB
C
D
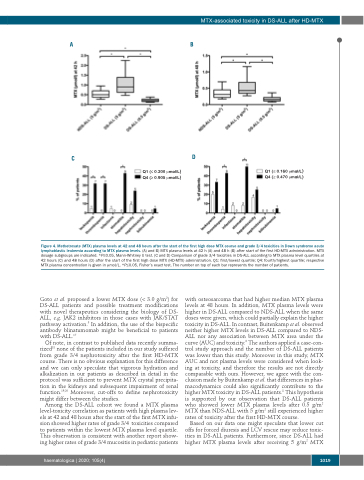
Q1 (≤ 0.200 μmol/L) Q4 (≥ 0.905 μmol/L)
Q1 (≤ 0.160 μmol/L) Q4 (≥ 0.470 μmol/L)
Figure 4. Methotrexate (MTX) plasma levels at 42 and 48 hours after the start of the first high dose MTX course and grade 3/4 toxicities in Down syndrome acute lymphoblastic leukemia according to MTX plasma levels. (A) and B) MTX plasma levels at 42 h (A) and 48 h (B) after start of the first HD-MTX administration. MTX dosage subgroups are indicated. *P≤0.05, Mann-Whitney U test. (C and D) Comparison of grade 3/4 toxicities in DS-ALL according to MTX plasma level quartiles at 42 hours (C) and 48 hours (D) after the start of the first high dose MTX (HD-MTX) administration. Q1: first/lowest quartile; Q4: fourth/highest quartile; respective MTX plasma concentration is given in μmol/L. *P≤0.05, Fisher’s exact test. The number on top of each bar represents the number of patients.
Goto et al. proposed a lower MTX dose (< 3.0 g/m2) for DS-ALL patients and possible treatment modifications with novel therapeutics considering the biology of DS- ALL, e.g. JAK2 inhibitors in those cases with JAK/STAT pathway activation.5 In addition, the use of the bispecific antibody blinatumomab might be beneficial to patients with DS-ALL.17
Of note, in contrast to published data recently summa- rized18 none of the patients included in our study suffered from grade 3/4 nephrotoxicity after the first HD-MTX course. There is no obvious explanation for this difference and we can only speculate that vigorous hydration and alkalization in our patients as described in detail in the protocol was sufficient to prevent MTX crystal precipita- tion in the kidneys and subsequent impairment of renal function.19,20 Moreover, cut-offs to define nephrotoxicity might differ between the studies.
Among the DS-ALL cohort we found a MTX plasma level-toxicity correlation as patients with high plasma lev- els at 42 and 48 hours after the start of the first MTX infu- sion showed higher rates of grade 3/4 toxicities compared to patients within the lowest MTX plasma level quartile. This observation is consistent with another report show- ing higher rates of grade 3/4 mucositis in pediatric patients
with osteosarcoma that had higher median MTX plasma levels at 48 hours. In addition, MTX plasma levels were higher in DS-ALL compared to NDS-ALL when the same doses were given, which could partially explain the higher toxicity in DS-ALL. In contrast, Buitenkamp et al. observed neither higher MTX levels in DS-ALL compared to NDS- ALL nor any association between MTX area under the curve (AUC) and toxicity.8 The authors applied a case-con- trol study approach and the number of DS-ALL patients was lower than this study. Moreover in this study, MTX AUC and not plasma levels were considered when look- ing at toxicity, and therefore the results are not directly comparable with ours. However, we agree with the con- clusion made by Buitenkamp et al. that differences in phar- macodynamics could also significantly contribute to the higher MTX toxicity in DS-ALL patients.8 This hypothesis is supported by our observation that DS-ALL patients who showed lower MTX plasma levels after 0.5 g/m2 MTX than NDS-ALL with 5 g/m2 still experienced higher rates of toxicity after the first HD-MTX course.
Based on our data one might speculate that lower cut offs for forced diuresis and LCV rescue may reduce toxic- ities in DS-ALL patients. Furthermore, since DS-ALL had higher MTX plasma levels after receiving 5 g/m2 MTX
haematologica | 2020; 105(4)
1019