Page 235 - Haematologica March 2020
P. 235
CXCR4-targeted nanocarrier to DLBCL cells
icantly increased the number of apoptotic bodies and cleaved PARP level compared to buffer-treated mice (Figure 8A and B).
We then confirmed CXCR4 expression in hematopoietic cells of the mouse BM (CXCR4+ CD20- staining) (Figure
8C). A direct comparison showed that CXCR4 expression in SC Toledo tumors was significantly (22.87 times) higher than CXCR4 in mouse BM hematopoietic cells (Online Supplementary Figure S2C and D). No histopathological alterations (H&E) nor induction of cell death (DAPI stain-
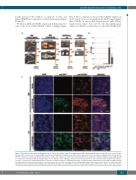
Figure 7. T22-GFP-H6 nanocarrier biodistribution in the Toledo-Luci diffuse large B-cell lymphoma (DLBCL) disseminated mouse model. (A) Level of fluorescence intensity (FLI) emission in bone marrow (BM) (cranium and hind limbs) and lymph nodes (LN) (cervical and renal) 5 hours (h) after the administration of 400 mg T22- GFP-H6 or buffer in a Toledo-Luci disseminated mouse model. (B) Comparison of FLI emission by T22-GFP-H6 accumulated in infiltrated DLBCL organs (BM and LN) as compared to non-DLBCL infiltrated organs (spleen, liver, kidneys, heart and lungs). FLI ratio from experimental mice was calculated subtracting the FLI auto-fluo- rescence of control mice and dividing the FLI recorded for each tissue by the FLI emitted by the lungs. (C) Representative immunofluorescent images of BM (cranium) and LN (cervical) in nanocarrier-treated mice and buffer-treated mice. Notice that green dots depicting internalized nanocarrier in the cytosol are only observed in T22-GFP-H6 treated animals. DAPI staining (blue), anti-GFP protein (green), anti-CXCR4 receptor (red) and merged images from the three stains. Scale bars=10 mm.
haematologica | 2020; 105(3)
749
A
B
C