Page 232 - Haematologica March 2020
P. 232
A. Falgàs et al.
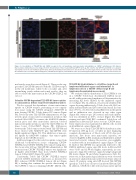
Figure 4. Co-localization of T22-GFP-H6 and CXCR4 receptor in the cell membrane and nanocarrier internalization in CXCR4+ subcutaneous (SC) tumors.
Representative immunofluorescence images from SC Toledo tumors of mice treated with T22-GFP-H6 (200 mg, 5h) or buffer. T22-GFP-H6 and CXCR4 co-localization was mainly seen in the cell membrane (yellow dots), whereas internalized nanocarriers were observed in the cell cytosol (green dots) and the endocytic vesicles with the CXCR4 receptor (red dots). DAPI staining (blue), anti-GFP protein (green), anti-CXCR4 receptor (red) and merged images from the three stains. Scale bars=10 mm.
mal vesicles were dissociated (Figure 4). These results sug- gest that T22-GFP-H6 interacts with the CXCR4 receptor in the cell membrane, where both co-localize and, after internalizing jointly within endosomal vesicles, they are able to release the nanocarrier in the CXCR4+ DLBCL cell cytosol.
Selective CXCR4-dependent T22-GFP-H6 tumor uptake in subcutaneous diffuse large B-cell lymphoma tumors
We also assessed the dependence of nanocarrier tumor uptake on CXCR4 receptor, performing in vivo competi- tion assays using the CXCR4 antagonist AMD3100 in mice bearing CXCR4+ Toledo-derived SC tumors (Figure 5A). Five hours after T22-GFP-H6 administration, we reg- istered a peak of nanocarrier accumulation in tumors that reached 3.23±0.38E7. In contrast, the AMD3100 adminis- tration prior and after nanocarrier injection blocked nanocarrier uptake in tumors, since the emitted FLI was 10 times lower (0.31±0.52E7) (Figure 5B). Differences between the Toledo tumors treated with T22-GFP-H6 and those treated with AMD3100 plus T22-GFP-H6 were highly significant (Figure 5C). This inhibition of nanocar- rier uptake by AMD3100 confirms that tumor uptake depends on the CXCR4-receptor.
Additional support for this selective uptake comes from additional biodistribution assays comparing CXCR4– SUDHL-2 and CXCR4+ SUDHL-2 SC tumor-bearing mice. Five hours after 200 mg T22-GFP-H6 administration, FLI emission from CXCR4+ SUDHL-2 tumors was significant- ly higher (2.12±0.46E7) than from CXCR4– SUDHL-2 tumors (0.04±0.21E7) (Figure 5C and D).
Consistently, Toledo and CXCR4+ SUDHL-2 tumors showed CXCR4 membrane expression, as measured by IHC, whereas CXCR4– SUDHL-2 tumors did not (Figure 5E); a finding that confirms the specific directioning of T22-GFP-H6 to tumors containing CXCR4+ DLBCL cells.
T22-GFP-H6 biodistributes to all diffuse large B-cell lymphoma-infiltrated organs and internalizes in lymphoma cells in a CXCR4+ diffuse large B-cell lymphoma disseminated mouse model
We evaluated the biodistribution of T22-GFP-H6 in vivo in a CXCR4+ Toledo-Luci disseminated DLBCL mouse model, while monitoring lymphoma cell dissemination by measuring BLI levels emitted by the infiltrated organs in vivo (Figure 6A). In addition, we precisely identified the organs showing infiltration by Toledo-Luci cells, BM (cra- nium and hind limbs) and LN (cervical and renal). In some mice (37.5%), we detected residual BLI levels in the spleen and no infiltration was observed in any other organ (Figure 6B). Macroscopic LN (cervical and renal) infiltra- tion was identified in 100% of mice (Figure 6C). H&E staining and anti-CD20 IHC confirmed Toledo-Luci cell infiltration in BM and LN tissue sections. CXCR4 mem- brane expression was maintained in DLBCL cells located in all infiltrated organs (Figure 6D).
We went on to study T22-GFP-H6 biodistribution after IV injection (400 mg dose) or buffer in mice displaying complete dissemination of Toledo cells (27-30 days post injection). Five hours after nanocarrier injection, we observed high FLI in BM (cranium and hind limbs) and LN (renal and cervical), whereas fluorescence was negligible or undetectable in non-infiltrated organs (Figure 7A and B). Indeed, T22-GFP-H6 was specifically delivered to the DLBCL infiltrated organs since FLI levels in BM and LN were 31.05- and 12.98-fold higher, respectively, in com- parison to lungs (the reference organ showing background FLI levels) (Figure 7B and Online Supplementary Table S3). Moreover, no histopathological alterations were observed in any tissue analyzed in nanocarrier-treated mice (data not shown). IF analysis using anti-GFP showed T22-GFP-H6 (green) in Toledo-Luci cell cytosol in affected BM and LN. In addition, CXCR4 dot-like (red) and nanocarrier (green)
746
haematologica | 2020; 105(3)