Page 224 - Haematologica March 2020
P. 224
C. Martín-Cortázar et al.
edge and its detachment at the opposite end of the cell, amoeboid movement is driven by short-lived and relative- ly weak interactions with the ECM.7 In amoeboid migra- tion, movement is generated by cortical filamentous actin in the cell front in the absence of focal contacts and stress fibers.7
Given that the lymphoma cells used in our study do not express MMP2 and MMP9 and bind fibronectin very
weakly, we propose that these cells use an amoeboid type of invasion. In line with a minor role for cell adhesion in the movement of these cells, the inhibition of cell migra- tion and invasion upon CDCA7 silencing was not paral- leled by a substantial modification of their binding to fibronectin. Accordingly, the expression and activity of VLA-4, the major fibronectin receptor of these cells, was not affected by CDCA7 knockdown.
AC
B
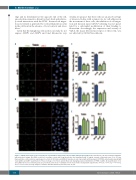
Figure 7. CDCA7 silencing decreases tropomyosin 3 polarization and promotes myosin light chain phosphorylation. BL-2 and Toledo cells were transduced with the indicated short hairpin (sh) RNA, seeded on coverslips coated with 2 μg fibronectin, and stimulated with 10 ng/mL stromal cell-derived factor 1 for 15 min. Representative confocal microscopy images (1 section) of (A) Toledo and (B) BL-2 transduced cells stained with anti-tropomyosin 3 (TPM3), anti-phospho-myosin light chain (pMLC) and DAPI. Quantification of the percentage of (C) Toledo and (D) BL-2 cells displaying polarized distribution of TPM3 or pMLC. (E) Quantification of rel- ative pMLC fluorescence intensity. Data are presented as the mean + standard error of mean of three independent experiments. *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001 (one-way analysis of variance with the Bonferroni post-test). Bar, 10 mm.
E
D
738
haematologica | 2020; 105(3)