Page 169 - Haematologica March 2020
P. 169
Modeling chronic myeloid leukemia in zebrafish
A
B
C
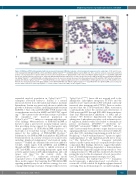
Figure 5. Wild-type (WT) fish transplanted with chronic myeloid leukemia (CML)-like leukemic cells show myeloid expansion in the early stage. Of 43 and 65 recip- ients transplanted with Tg(lyz:DsRed) control and CML-like Tg(hsp70:p210BCR/ABL1-lyz:DsRed) kidney marrow (KM) cells, three and four survived, respectively. (A) DsRed positive cells repopulated in recipients within 2-3 weeks after transplantation of Tg(lyz:DsRed) control (left) and CML-like Tg(hsp70:p210BCR/ABL1-lyz:DsRed) (right) KM blood cells. (B) All survived recipients were stained by May-Grunwald-Giemsa. Myelocytes (d, blue arrows) increased in KM of WT fish after transplanted with CML- like Tg(hsp70:p210BCR/ABL1-lyz:DsRed) KM cells. Myeloid precursors (b and d, red arrows) increased in both peripheral blood (PB) and KM of WT fish after being trans- planted with CML-like Tg(hsp70:p210BCR/ABL1-lyz:DsRed) KM blood cells. Original magnification x400. (C) hBCR/ABL1+ cDNA fragments detected in PB and KM cells of WT fish after being transplanted with Tg(lyz:DsRed) control (WT-PB/WT-KM) and CML-like Tg(hsp70:p210BCR/ABL1-lyz:DsRed) KM cells (Tg-PB/Tg-KM) assessed by poly- merase chain reaction. PC: pToL hsp70:p210BCR/ABL1 as the positive control. NC: ddH2O as the negative control.
expanded myeloid population in Tg(hsp70:p210BCR/ABL1) transgenic zebrafish embryos. Icaritin is a natural flavonoid derived from the traditional Chinese medicine Epimedium. Icaritin was previously shown to inhibit the growth of leukemic cell lines, including imatinib-resistant BCR/ABL1+ blast cells and BCR/ABL1-T315I mutant cells via mechanisms involved in MAPK and JAK/STAT signal- ing.27-29 The current results show that icaritin could reduce the expanded lcp1+ myeloid population in Tg(hsp70:p210BCR/ABL1) embryos, consistent with these pre- vious findings. Overactivation of PI3K/AKT/mTOR is known to play a pivotal role in many human cancers, thus providing strong support for the therapeutic anti- cancer application of PI3K/Akt/mTOR inhibitors.30,31 Sadovnik et al. found that escape of CML LSC was dis- rupted by the addition of PI3K/mTOR blockers.32 Furthermore, the PI3K/mTOR dual inhibitor BEZ235 had beneficial effects on a variety of tumors in vivo and in vitro, including lymphoid malignancies33 and myeloid malig- nancies.34,35 Bendell also identified the active-site inhibitor CC-223, which targets both mTORC1 and mTORC2 through mTOR kinase activity to inhibit activation of AKT and 4EBP1, as a promising therapeutic agent with activity against many non-Hodgkin lymphoma and solid tumor cell lines.36 In the current study, the Tg(hsp70:p210BCR/ABL1) embryonic zebrafish model responded well to both BEZ235 and CC-223. Overall, these results suggest that targeting the PI3K/Akt/mTOR signaling pathway may be an effective strategy for over- coming CML therapy resistance. Unexpectedly, however,
Tg(hsp70:p210BCR/ABL1) larvae did not respond well to the sphingosine 1-phosphate antagonist, FTY720, and the number of lcp1+ myeloid cells in WT zebrafish conversely increased after treatment with FTY720. Previous studies reported that the FTY720-mediated PP2A reactivation could markedly reduce the survival and self-renewal of CML-quiescent HSC through BCR-ABL1 kinase-indepen- dent and PP2A-mediated inhibition of JAK2 and β- catenin.37 We therefore hypothesized that, although sphingosine 1-phosphate may play a role in hematopoiet- ic regulation, further studies are needed to determine its precise mechanism. LY364947,38,39 ciliobrevin A,40 DB07268,41 selonsertib,42 NQDI-1,43 AZD3759,44 and ico- tinib45 have recently been shown to target key factors and signaling pathways essential for the survival of CML LSC and other CSC, including transforming growth factor β,39 Hedgehog,46 c-Jun N-terminal kinases,47 apoptosis signal- regulating kinase 1,48 and epidermal growth factor recep- tor.49 The Tg(hsp70:p210BCR/ABL1) transgenic zebrafish embryos in the current study also responded well to these compounds. Our findings, therefore, suggest that inhibi- tion of BCR/ABL1 kinase-dependent or kinase-indepen- dent pathways (Figure 6D) might offer potential for over- coming resistance to TKI and thus eradicate LSC, thereby paving the way for the development of novel, more effec- tive LSC-eradicating treatment strategies for CML.
In conclusion, the Tg(hsp70:p210BCR/ABL1) transgenic model represents a phenotype-based, cost-effective, in vivo model of CML suitable for high-throughput chemical screening. This model may improve our understanding of
haematologica | 2020; 105(3)
683