Page 270 - 2020_02-Haematologica-web
P. 270
S. Staessens et al.
Interestingly, leukocytes have been shown to promote thrombus formation by the formation of extracellular DNA traps. We and others recently described the presence of neutrophil extracellular DNA traps (NET) in acute ischemic stroke thrombi.14,15 To further examine the pres- ence and internal organization of DNA networks in stroke thrombi, we performed a highly sensitive Feulgen’s DNA staining on a subset of 100 stroke thrombi. Strikingly, large extracellular DNA networks, appearing as extracellu- lar smears, were seen throughout the majority of thrombi. Again, abundant amounts of extracellular DNA were observed particularly in the platelet-rich areas and in the boundary areas between platelet-rich and RBC-rich regions (Figure 7). No extracellular DNA was found within the RBC-rich regions (Figure 7E). In conclusion, leukocytes and networks of extracellular DNA were found specifical- ly on the interface of the platelet-rich and RBC-rich regions, and in the platelet-rich regions.
Discussion
This study provides a detailed description of composi- tional features of ischemic stroke thrombi. We found that
stroke thrombi consist of RBC-rich and platelet-rich areas. RBC-rich areas have a limited complexity and are com- posed of densely packed RBC that are contained in a mesh- work of thin fibrin, with a small number of leukocytes that are spread homogeneously throughout the RBC. In con- trast, platelet-rich areas are more complex and contain var- ious scaffolds that include fibrin, vWF and DNA. Dense fib- rin structures are aligned with vWF and are packed with platelets. In addition, leukocytes and DNA tend to accumu- late within the platelet-rich areas and on the interface between platelet- and RBC-rich areas. Why different parts of the same thrombus have such distinct underlying archi- tecture is an intriguing point. Local hemodynamic forces are known to regulate the thrombotic pathways and thus the biochemical make-up of thrombi. Interestingly, also throm- bus contraction, which is mediated by contractile forces of platelets on fibrin, has been suggested to mediate spatial separation of RBC and platelet aggregates.16 Thrombus con- traction leads to the compression of RBC to so-called poly- hedrocytes, forming large clusters of densely packed RBC, and to redistribution of platelets to the exterior.16 Although our histology does not allow us to unequivocally identify the typical convex, irregular polyhedral structure of com- pressed RBC, some confocal images (e.g. Figure 3C) are
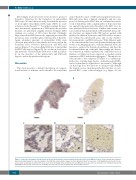
AB
CDE
Figure 6. Leukocytes accumulate in platelet-rich areas and at the interface between platelet-rich and red blood cell (RBC)-rich areas. Stroke thrombi were immuno- histochemically analyzed for leukocytes (purple). (A and B) Two representative images of stroke thrombi stained for leukocytes. (C-E) Magnifications show that leuko- cytes tend to accumulate in platelet-rich areas (C) or at the boundary between platelet-rich and RBC-rich areas (D), whereas leukocytes are homogenously spread within RBC-rich areas (E). Scale bars are: (A and B) 500 μm; (C-E) 100 μm. P: platelet-rich area; R: RBC-rich area.
504
haematologica | 2020; 105(2)