Page 271 - 2020_02-Haematologica-web
P. 271
Structural hallmarks of stroke thrombi
reminiscent of polyhedrocytes observed in contracted thrombi.16,17 Thrombus contraction was reported to be reduced in patients with ischemic stroke, but could have profound effects on thrombus organization, thrombus vol- ume (and thus blood flow past thrombo-embolic occlu- sions), and thrombus density.18 More studies are needed to fully understand the potential effect of thrombus contrac- tion in ischemic stroke patients.
In general, our findings are important to advance our understanding of ischemic stroke pathophysiology and, more importantly, to guide the development of better recanalization strategies in stroke patients via thromboly- sis or thrombectomy.
As far as thrombolysis is concerned, recombinant tissue plasminogen activator is currently the only FDA-approved drug for pharmacological thrombolysis of ischemic stroke thrombi. However, it is only effective in less than half of the patients that receive rt-PA.2,3 The mechanisms under- lying this so-called "rt-PA-resistance" are not completely understood, but previous reports indicated that RBC-dom- inant thrombi respond better to rt-PA than platelet-domi- nant thrombi.19-27 Our histological findings provide new molecular insights on the composition of RBC-rich and platelet-rich thrombus material. It seems plausible that rt-
PA, which promotes the degradation of fibrin, can have a direct and efficient thrombolytic effect on the RBC-rich areas in which thin fibrin is the main extracellular scaffold. In contrast, however, our histological analyses reveal that platelet-rich thrombus material contains denser fibrin structures that also include vWF. In addition, we show that platelet-rich areas comprise substantial amounts of extracellular DNA. Therefore it is tempting to speculate that vWF and DNA, together with fibrin, form the struc- tural basis of platelet-rich thrombi, and that vWF and DNA, at least partially, could be responsible for the observed rt-PA-resistance of platelet-rich thrombi in patients.
Extracellular DNA and histones have indeed been shown to modify the structure of fibrin, making it more resistant to enzymatic degradation via rt-PA.28 The exact source of DNA observed in our study remains to be inves- tigated, but neutrophils support thrombosis via the forma- tion of neutrophil extracellular DNA structures that can act as a thrombogenic scaffold.29-31 Importantly, ex vivo thrombolysis experiments have shown that rt-PA in com- bination with DNAse-1 is more effective than rt-PA alone, further underlining the potential importance of extracellu- lar DNA for rt-PA-resistance.14,15
AB
CDE
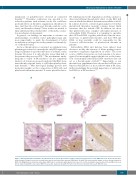
Figure 7. Extracellular DNA accumulates in platelet-rich areas and on the interface between platelet-rich and red blood cell (RBC)-rich areas. Stroke thrombi were stained using a Feulgen’s reaction to visualize intra (nuclei) and extracellular (smears) DNA (pink). (A and B) Two representative images of stroke thrombi stained for DNA. (C-E) Magnifications show that extracellular DNA tends to accumulate within platelet-rich areas (C) or at the boundary between platelet-rich and RBC-rich areas (D). No extracellular DNA is observed in RBC-rich areas (E). Scale bar: (A and B) 500 μm; (C-E) 100 μm. P: platelet-rich area; R: RBC-rich area.
haematologica | 2020; 105(2)
505