Page 147 - 2020_02-Haematologica-web
P. 147
RKIP loss aggravates RAS-driven leukemogenesis
(HSPC RKIP KD) as compared to HSPC control KD (P=0.001 for CD11b+ cells and P=0.002 for CD11b+ CD14+ cells) (Figure 2A), confirming a role of RKIP loss in myeloid differentiation of HSPC. RKIP KD as a single event with- out the addition of cytokines was insufficient to induce myelomonocytic differentiation (data not shown). Of note, RKIP is a negative regulator of RAS-MAPK/ERK signaling. To evaluate whether RKIP KD indeed enhances RAS-MAPK/ERK signaling in HSPC, we studied the phos- phorylation of ERK (pERK) in the conditions mentioned above. Indeed, HSPC with RKIP KD displayed increased pERK levels (Figure 6A), suggesting that the RKIP-induced aggravation of differentiation might be mediated via acti- vation of the RAS-MAPK/ERK pathway.
To further corroborate the role of RKIP in myelomono- cytic differentiation, we again employed the 1,25D3-based HL-60 differentiation model and performed additional RKIP modulation by transfection of RKIP siRNA and over- expression constructs, respectively (Online Supplementary Figure S2). Initially, we thereby sought to confirm the role of RKIP on RAS-MAPK/ERK signaling and therefore stud- ied the expression of pERK. In agreement with the results from healthy CD34+ HSPC, RKIP KD thereby increased pERK levels, which corroborates the role of RKIP as a reg- ulator of RAS-MAPK/ERK signaling (Figure 6B). To study the role of RKIP on myelomonocytic differentiation, we assessed the CD11c surface expression in these experi- ments, which was previously established as the most suit- able marker for this HL-60-based experimental approach.36 1,25D3-treated HL-60 cells harboring RKIP knockdown thereby demonstrated an increased potential to differenti- ate into the myeloid lineage as assessed by an increase in the expression of CD11c (P=0.009) (Figure 2B). The oppo-
site effect was observed after RKIP overexpression (P=0.005) (Figure 2C). Finally, we studied the effect of RKIP modulation without additional 1,25D3 incubation. As seen for healthy HSPC, myelomonocytic differentia- tion could not be induced in this scenario (P=0.112 for RKIP knockdown and P=0.168 for RKIP overexpression).
Rkip deletion contributes to the development of a myelomonocytic-lineage-biased hematopoietic system in a murine in vivo model
After demonstrating the functional involvement of RKIP loss in myelomonocytic differentiation in vitro, we focused on its effects in vivo. To do this, we analyzed the hematopoietic system in a murine model with a complete deletion of Rkip (Rkip-/-) (Online Supplementary Table S1 and Online Supplementary Figures S3 and S4). In agreement with the in vitro data, RKIP loss as a single event thereby proved insufficient to increase the amount of myelomonocytic cells. Rkip deletion alone further proved insufficient to increase the proliferation of specific HSPC compartments (Online Supplementary Figure S4). As our in vitro data already suggested that RKIP acts as a modulator for the sensitivity to extracellular inducers of differentiation, we aimed to challenge the hematopoietic system of Rkip-/- mice by the additional intraperitoneal injection of GM- CSF in a next step (Figure 3A). Indeed, Rkip-/- mice demon- strated an increased percentage of CD11b+ as well as CD11b+ Ly6G+ myelomonocytic cells in bone marrow, peritoneal cavity cells and peripheral blood in these exper- iments (Figure 3B and C). As seen in the in vitro experi- ments mentioned above, Rkip deletion also enhanced RAS-MAPK/ERK signaling, as evidenced by increased pERK levels in Rkip-/-mice (Figure 6C). Taken together, our
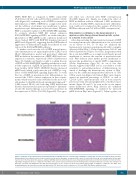
Figure 5. Induction of myeloproliferation in Nras-mutated mice with Rkip deletion coincides with a mitigation of histiocytic sarcoma development. Representative Hematoxylin & Eosin stained sections of spleen and liver of the mouse genotypes as indicated. Mice were electively killed at an age of six months after the first pIpC injection. As in the flow cytometric analyses, animals with Rkip deletion demonstrated increased myeloproliferation (spleen, bottom left and insert bottom left show- ing multiple megakaryocytes) as compared to the Rkip+/+ mice (spleen top left, and insert top left showing almost exclusively histiocytic sarcoma). The formation of histiocytic sarcomas was mitigated in the Rkip deleted genotypes as also clearly seen in the liver sample (bottom right, no infiltrate) compared to the Rkip+/+ liver showing infiltration by histiocytic sarcoma (top right, arrow). The black bar denotes a distance of 500 μm. RKIP: RAF kinase inhibitor protein.
haematologica | 2020; 105(2)
381