Page 172 - 2019_12-Haematologica-web
P. 172
S.N. Chaurasia et al.
ly, concurrent with the synthesis of HIF-2a. Puromycin (10 mM) significantly attenuated PAI-1 expression in hypoxic cells (Figure 3A, D) as well as in hypoxia-mimet- ic-treated cells (Figure 3B, E). A prothrombotic phenotype associated with hypoxia may, therefore, be at least in part attributable to synthesis of PAI-1 by platelets under hypoxic stress.
PEV are membrane-bound cellular fragments ranging in size from 0.1 to 1 μm that are shed by stimulated or stressed platelets.31,32 PEVs are endowed with pro-coagu- lant properties and play a vital role in hemostatic respons- es.33,34 Exposure of platelets to hypoxia for 2 h led to exten- sive shedding of PEV, in numbers 1.5- to 3.0-fold higher than those released from normoxic counterparts under similar conditions (Figure 3C). Thus, it can be surmised that shedding of PEV together with production of PAI-1 by platelets would contribute significantly to a thrombo- genic state in a hypoxic environment. In order to implicate hypoxia-induced platelet signaling in the pathogenesis of arterial thrombosis in vivo, we studied the effect of hypox- ia-mimetics in a murine model of ferric chloride-induced mesenteric arteriolar thrombosis. Remarkably, as shown in Figure 3F, mice pretreated with DMOG (400 mg/kg) (Online Supplementary Video 1) or DFO (200 mg/kg) (Online
AB
Supplementary Video 2) were found to exhibit significantly accelerated thrombus formation compared to that of con- trol mice (Online Supplementary Video 3) (mean time to form first thrombus: DMOG, 7.16 ± 0.66 min; DFO, 7.0 ± 0.86; control, 9.6 ± 0.16 min). Administration of hypoxia- mimetics also evoked an increase in thrombus growth rate in mice (Figure 3H) although the mean times to occlusion were not significantly different (Online Supplementary Figure S2). These results strongly suggest that platelet hypoxia signaling induces a prothrombotic state in vivo.
Hypoxia-mimetics induce shedding of extracellular vesicles and a rise in intracellular free calcium in human platelets
As hypoxic stress led to the release of EV from human platelets (Figure 3C), we examined the effect of the hypoxia-mimetics DMOG and DFO, which stabilize HIF- a subunits by inhibition of prolyl hydroxylases, on platelets. Exposure of platelets to either DMOG (1 mM) or DFO (1 mM) for 15 min at 37°C in a normoxic environ- ment led to significantly higher expression of HIF-2a (by 37% and 57.7%, respectively) compared to that of the control platelets (Figure 4A, B). Remarkably, both hypox- ia-mimetics induced significant shedding of EV from
C
DEF
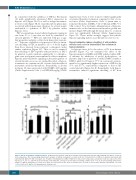
Figure 2. HIF-2a degradation in human platelets. (A) Western blot analysis of HIF-2a in platelets treated with either PSI (50 μM) or MG132 (50 μM) for 30 min at room temperature. (D) Corresponding densitometric analysis of HIF-2a normalized to β-actin (n=4). (B, C) Western blots of HIF-2a in platelets pretreated with bafilomycin A1 (BAF, 250 nM), chloroquine (CQ, 50 μM) or 3-methyladenine (3-MA, 5 mM) for 30 min at room temperature as indicated. Cells were exposed to either hypoxia (1% O2, 5% CO2, and 94% N2) for 30 min (B), or thrombin (Thr, 1 U/mL) for 10 min under non-stirring condition at 37°C (C). (E, F) Corresponding densitometric analyses of HIF-2a normalized to β-actin (n=5). Data are represented as the mean ± standard error of mean of at least three different experiments. *P<0.05; **P<0.01; #P<0.05; ##P<0.01, analyzed by the Student t test.
2486
haematologica | 2019; 104(12)