Page 54 - 2019_09-HaematologicaMondo-web
P. 54
Z. Zhang et al.
ABC
DEF
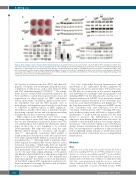
Figure 1. Role of adipose stem cell factor (SCF) in brown fat function. (A and B) Stromal vascular faction (SVF) cells from iWAT of Kitlfl/fl and Adipoq-Cre+;Kitlfl/fl mice were differentiated into adipocytes in vitro and analyzed by Oil O Red staining (A) and western blotting (B). Densitometry of UCP1 shown in Online Supplementary Figure S1C. (C and D) Expression of proteins involved in adipogenesis and thermogenesis in BAT (C) and iWAT (D) from 7-week old Kitlfl/fl and Adipoq-Cre+;Kitlfl/fl male mice. Densitometry of UCP1 shown in Online Supplementary Figure S1D and E. (E and F) Kitlfl/fl and Adipoq-Cre+;Kitlfl/fl mice (n=6) were treated with CL 316,243 for seven days. Ucp1 mRNA levels (E) and UCP1 protein levels (F) in BAT and iWAT were determined. Densitometry of UCP1 shown in Online Supplementary Figure S1J and K. Data are presented as mean±Standard Deviation.
also known as skeletal stem cells (SSC)] and their adi- pogenic, osteogenic, and chondrogenic progeny are major contributors of niche factors, such as stem cell factor (SCF) and CXC chemokine ligand 12 (CXCL12).20-22 The sympa- thetic nervous system (SNS) extensively innervates the bone and BM to control hematopoietic homeostasis and regeneration via direct actions on HSPC and indirect actions on the niche.23 In addition, signals from the vascu- lar endothelial cells and the HSC progeny such as macrophages and megakaryocytes have also been shown to contribute to different aspects of HSPC regulation.17 Nevertheless, whether these niche constituents mediate the sensing of metabolic cues and subsequent remodeling in hematopoiesis has not yet been determined.
White adipose tissue (WAT) that stores excess energy and brown adipose tissue (BAT) that dissipates energy as heat are key determinants of metabolic homeostasis. The role of BM adipose tissue (MAT), the third major adipose depot in the body, is just beginning to be revealed. Developmentally, BM adipocytes arise from the same Osterix+ skeletal lineage as osteoblasts and chondro- cytes.24-26 Anatomically, constitutive MAT (cMAT) is found in the most distal portion of the tibia and tail verte- brae while regulated MAT (rMAT) is found in the proxi- mal skeletal sites.27-29 Although cMAT is relatively stable, rMAT expands in conditions like obesity, diabetes, caloric restriction, and aging.27-29 Functionally, there are tripartite interactions between MAT, bone, and hematopoiesis, yet their mechanistic characteristics are still not fully under- stood.30 An early study taking advantage of the genetic and pharmacological inhibition of adipogenesis suggested MAT to be a negative regulator of the hematopoietic microenvironment.31 In contrast, recent work demonstrat- ed that MAT supports HSC regeneration and myeloery- throid maturation following irradiation and reconstitu- tion, partially by secreting SCF.32,33
The close relationship between hematopoiesis and metabolism is also represented by their regulation by common growth factors and cytokines. SCF and its recep- tor KIT play an essential role in the survival, migration, and differentiation of multiple stem and progenitor cells including HSPC.34 In the hematopoietic system, loss-of- function mutations in SCF/KIT cause macrocytic anemia while gain-of-function mutations lead to systematic mas- tocytosis, acute myeloid leukemia, and lymphoma.35,36 In the bone marrow niche, SCF is expressed in LEPR+ stroma cells, endothelial cells, and adipocytes, but not in osteoblasts or hematopoietic cells.22,32 Deleting SCF selec- tively in these positive niche cells leads to defects in HSC maintenance.22,32 In the metabolic system, SCF has been shown to promote the differentiation of brown adipocytes from human pluripotent stem cells and to be essential to mitochondrial function and energy expendi- ture in mice.37,38 However, the cellular source of SCF in reg- ulating systemic metabolism has not been determined. Here, we investigated the contribution of adipose-derived SCF in regulating energy and glucose metabolism and in mediating the effect of metabolic stresses on unperturbed hematopoiesis.
Methods
Mice
All mice used in this study were purchased from the Jackson Laboratory, including Kitlfl/fl (stock n. 017861), Adipoq-Cre (stock n. 010803), Osx1-Cre (stock n. 006361), and KitlEGFP (stock n. 017860). All animals were kept on a 14 hour (h):10 h light:dark cycle in the animal facility at the University of Minnesota, Minneapolis, MN, USA. Mice were group-housed, with free access to water and either a standard chow diet or 60% high fat diet (Research Diets, D12492). 1 mg/kg BW of CL-316, 243 (R&D Systems, #1499,
1732
haematologica | 2019; 104(9)