Page 181 - 2019_08-Haematologica-web
P. 181
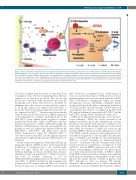
ATRA protects impaired BM MSC in ITP
Figure 7. Proposed model for aberrantly enhanced complement activation and interleukin-1β expression in mesenchymal stem cells from patients with immune thrombocytopenia. C5b-9 deposition and the associated pro-inflammatory cytokine interleukin-1β (IL-1β) could induce dysfunction of bone marrow mesenchymal stem cells (MSC) in immune thrombocytopenia (ITP), and consequently cause dynamic changes in niche CXCL12 and alterations in the location of megakaryocytes in the bone marrow. All-trans retinoic acid (ATRA) has an inhibitory effect on IL-1β mRNA expression and increases promoter DNA methylation, further promoting in vitro and in vivo functional recovery of MSC from ITP patients with complement deposition on their MSC (MSC-ITP-C+), which facilitates the re-location of CXCL12 towards the vascular niche, and enhances megakaryocyte localization in thrombopoietic niche.
of IL-1β is complex with the release of functional IL-1β requiring two steps. The first, rate-limiting step is the tran- scription of its precursor, pro-IL-1β. The second step involves the conversion of the inactive precursor into the biologically active IL-1β. This process is mediated by inflammasomes that activate cysteine protease caspase- 1.45 IL-1β and caspase-1 are expressed by monocytes, as previously reported, and interestingly, these molecules are also expressed by MSC.24,25 The microarray data from this study showed an absolute increase in mRNA expression of pro-inflammatory cytokines, including IL-1β, in the MSC-ITP-C+ group. Further results suggested a comple- ment-IL-1β loop in MSC, which contributed to the dys- function and apoptosis of MSC in ITP. Interestingly, the numbers of hematopoietic stem cells and megakaryocyte progenitors were not significantly different between the MSC-ITP-C+, MSC-ITP-C- and MSC-control groups (Online Supplementary Figure S9). Whether MSC or osteoblasts (derived from MSC) are the primary target for attack by the complement-IL-1β loop in ITP remains to be elucidated.
Several studies have implicated chemokine CXCL12 sig- naling, through the CXCR4 receptor, in the maturational localization of megakaryocytes to the vascular niche. Some cell types within the bone marrow produce CXCL12, including osteoblasts, endothelial cells, and mes- enchymal stromal cell populations.46,47 Despite the grow- ing body of evidence indicating a role for CXCL12/CXCR4 in megakaryopoiesis, the effects of defi- cient MSC on CXCL12 distribution and megakaryocyte localization in ITP remain unknown. Here, we observed decreased expression of CXCL12 mRNA and protein in
MSC cell lysates, accompanied by an overall increase in bone-associated/central marrow CXCL12 and a decrease in the megakaryocytes associated with sinusoids in MSC- ITP-C+. In addition to enlarging the sample size, further investigations, such as establishing a transgenic murine model, would specifically help to identify the distribution of CXCL12 in the bone marrow niche. CXCR4 expression on megakaryocyte surfaces was higher in patients with ITP, with no difference between the MSC-ITP-C+ and the MSC-ITP-C- groups (Online Supplementary Figure S10).
ATRA has revolutionized the therapy of acute promye- locytic leukemia.26 ATRA also shows potential as an immune modulator, given that inflammatory cytokine production was affected in the presence of ATRA. In vitro studies have shown that natural regulatory T cells treated with ATRA are resistant to T-helper cell conversion and maintain FOXP3 expression under inflammatory condi- tions. ATRA could also promote transforming growth fac- tor-β-induced regulatory T cells and inhibit the differenti- ation of T-helper-1 and T-helper-17 cells, indicating that ATRA is a novel treatment for immune-mediated diseases.48,49 In patients with ITP, treatment with ATRA restored decreased concentrations of regulatory T cells and IL-10, reduced FOXP3 expression and restored the balance of macrophages towards M2.29 In addition, previ- ous studies demonstrated the ability of ATRA to inhibit IL-1β mRNA expression and its potential role in regulating DNA promoter methylation. Importantly, we previously reported the clinical efficacy of ATRA in patients with ITP,27 although its effect on primary ITP remains unknown. These findings led us to explore whether the mechanisms of action of ATRA in the treatment of ITP are
haematologica | 2019; 104(8)
1673