Page 112 - 2019_08-Haematologica-web
P. 112
S. Liu et al.
Discussion
Dysfunction of RUNX1 through gene mutations and chromosome translocations occurs frequently in various myeloid malignancies. As a transcription factor, RUNX1 exerts its biological effects through transcriptional regula- tion of its target genes. Thus, identification of RUNX1 tar- get genes and clarification of their roles would offer an intriguing insight into the mechanisms of how perturbed RUNX1 function may result in hematologic malignancies.
For this purpose, we comprehensively analyzed RUNX1 Chip-Seq data with our previous gene expression profiles of PB-treated Kasumi-1 cells. The result revealed a list of putative RUNX1 target genes which had a high potential
of driving differentiation and apoptosis of t(8;21) leukemia cells. These targets also served as potential candidates for developing new therapies to treat t(8;21) AML. Among them, p53-induced gene 7 (PIG7), a gene transactivated by RUNX1 through a specific RUNX1-binding site located in the promoter region (bp -1511 to -1503) and promoted apoptosis and differentiation of leukemia cells, has been reported.12 In the present study, we focused our attention on KLF4. On the basis of previously published studies, KLF4 is likely to have similar cellular phenotypes as those observed with RUNX1 in regulating cell proliferation and differentiation. It is reported that RUNX1 mediated inhi- bition of AML cell survival through repression of VEGF expression, a major mediator of angiogenesis, prolifera-
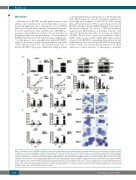
A
BC
D
F
E
Figure 6. Biological effects of RUNX1 and KLF4 overexpression on Kasumi-1 cell proliferation, apoptosis and differentiation. (A) Overexpression of RUNX1 and KLF4
in Kasumi-1 cells were mediated by a pCDH lentivirus system. At 72 hours (h) after infection, the infected cells were sorted by flow cytometry for GFP+ population and the expression levels of RUNX1 or KLF4 were measured by quantitative real-time polymerase chain reaction (left) and western blot (right), respectively. (B) MTS assay was performed to evaluate cell proliferation ability of GFP+ Kasumi-1 cells over-expressing RUNX1 or KLF4 at 72 h after lentivirus infection. (C) Cell cycle dis- tribution of GFP+ Kasumi-1 cells over-expressing RUNX1 or KLF4 was analyzed by flow cytometry with PI staining at 48 h after cell sorting. (D) Cell apoptosis analysis of GFP+ Kasumi-1 cells over-expressing RUNX1 or KLF4 were performed by the flow cytometry at 48 h and 72 h after lentivirus infection. (E) Morphological assess- ment of GFP+ Kasumi-1 cells over-expressing RUNX1 or KLF4 at indicated days after infection. Cells were stained by Wright-Giemsa staining and observed by oil microscopy after flow sorting (magnification x100). (F) Flow cytometry analysis of cell surface markers CD11b and CD15 of GFP+ Kasumi-1 cells over-expressing RUNX1 or KLF4 at 48 h and 72 h after lentivirus infection.
1604
haematologica | 2019; 104(8)