Page 60 - 2019_07 resto del Mondo-web
P. 60
M. Mazzola et al.
A A’ A’’ A’’’
B B’ B’’
C C’ C’’
D D’ D’’
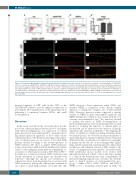
Figure 4. The canonical Wnt pathway is hyper-activated specifically in hematopoietic stem cells. (A-A’’) Fluorescence-activated cell sorting analysis of CD41:GFPlow cells from controls (A), nipblb-MO (A’) and NPMc+ mRNA (A’’) injected embryos at 3 days post-fertilization (dpf) and quantitative reverse transcriptase polymerase chain reaction analysis of axin2 expression on sorted cells (A’’’). (B-D’’) Immunofluorescence staining with green fluorescent protein (GFP) for hematopoietic stem cells. (B-D) and Active β-catenin for Wnt activation (Active β-cat) (B’-D’) antibodies. Merging of the two signals (B’’-D’’) showed an increased number of GFP/Active β- cat double-positive cells (arrows) at 3 dpf in the caudal hematopoietic tissue of embryos injected with nipblb-MO or NPMc+ mRNA in comparison to the number in controls. Images were processed as described in the Online Supplementary Methods. The scale bar represents 100 μm. *P<0.05. ns: non-significant; ctrl: control; MO: morpholino.
increased number of GFP+ cells in the CHT of the Tg(TOPdGFP) embryos and an enhanced expression of spi1b (Figure 6F-I, quantification of the phenotypes in J), indicating a cooperation between NPMc+ and nipblb downregulation.
Discussion
In this work, we built on the observation that, in addi- tion to the cohesin mutations detected in 10% of patients with myeloid malignancies, low expression of cohesin genes was present in an additional 15% of patients show- ing similar expression signatures as those with somatic cohesin mutations.28 We therefore investigated the expres- sion of cohesin genes in our cohort of 40 adult AML patients divided according to the absence/presence of NPM1 mutation. We chose to analyze NPM1 mutations as they are pivotal in AML but likely insufficient by them- selves to cause malignant transformation, requiring the co- occurrence of other mutations such as FLT3-ITD or RAS.9,34 In addition, a correlation between NPM1 mutations and somatic mutations in cohesin genes has already been reported,28 although the specific loss-of-function of cohesins has never been investigated in association with NPM1 mutations. Among the cohesin genes analyzed in our cohort of adult AML patients, we found that only
NIPBL showed a lower expression when NPM1 was mutated. NIPBL is a regulator of the cohesin complex deputed to loading the complex onto double-stranded DNA. However, previous studies suggested a specific activity of NIPBL in gene transcription regulation and NIPBL binding sites, which do not overlap with those of cohesins, were identified in vitro.35 We, therefore, decided to analyze the effects of NIPBL downregulation on myeloid differentiation. The zebrafish represents an ideal model for studying the function of cohesin genes as knockdown can be achieved by the injection of specific oligonucleotide antisense morpholino.30 Investigating the effects of nipblb-loss-of-function during zebrafish defini- tive hematopoiesis, we observed an increased number of myeloid progenitors. Similar results have been obtained in different models of cohesin loss-of-function. For example, in murine models the use of short hairpin RNA against Stag2 and Smc3 generated a maturation block, delayed dif- ferentiation, and enhanced renewal of HSC, similar to the events occurring in myeloid neoplasms.36 In another study, RNA interference mouse models were created with inducible knockdown of Rad21, Smc1a and Stag2, leading to a shift in the hematopoietic stem compartment, an increased replating capacity and, over time, the develop- ment of clinical features of myeloproliferative neo- plasms.18 Unlike STAG2, SMC3, SMC1A, and RAD21, NIPBL is not a recurrently mutated gene in AML.28 In
1338
haematologica | 2019; 104(7)