Page 171 - 2019_06-Haematologica-web
P. 171
Low TLT-1 and collagen receptor α2 in FPD/AML
A
B
C
adhesion to this substrate was severely impaired (Figure 5D and E). Moreover, platelet adhesion was also, albeit less markedly, reduced over fibrillar collagen I (Figure 5D and E). Considering that GPVI, which has a more promi- nent role over fibrillar collagen, was preserved, other abnormalities in FPD/AML platelets may contribute to defective adhesion to fibrillar collagen in addition to low α2b1. In contrast, platelet adhesion to convulxin, which relies on GPVI, and to fibrinogen, which depends mainly on GPIIbIIIa, were largely preserved (Online Supplementary Table S6). As an approach to mimic the in vivo conditions, we studied platelet aggregate formation under flow, which revealed a substantial decrease in surface area cov- erage over a collagen substrate for patient compared to control platelets (Figure 5F and G). Overall, these abnor- malities suggest that primary hemostasis might be impaired in FPD/AML, although the clinical relevance of this finding still has to be determined. On the other hand, defective collagen-induced aggregation (Table 1) cannot be explained only by decreased α2b1, as collagen prepara- tions used in aggregometry depend mainly on GPVI. Given this, the aggregation defect in response to collagen and other platelet agonists may also rely on the role played by TLT-1 in fibrinogen binding, platelet aggregate formation and stabilization.
Megakaryocytes in familial platelet disorder with predisposition to acute myelogenous leukemia display low levels of α2 integrin subunit and decreased adhesion to collagen I
Platelet production is a tightly regulated process gov-
erned by the close interaction between MK and bone mar-
row (BM) extracellular matrix (ECM) proteins. In addition
to its abundance at the vascular bed, collagen I is a crucial
component of the BM ECM. MK-collagen I interaction is
of critical importance in restraining proplatelet formation
at the osteoblastic niche, thus preventing premature
platelet release into the interstitial space and allowing nor-
mal platelet production into the lumen of BM sinusoids.33
Ligation of the α2b1 receptor in MK is required for stress
fiber formation and adhesion over collagen I, as shown in
both human and mouse MK,33,34 whereas although α2b1
integrin is involved in collagen I-induced inhibition of pro-
platelet formation in human MK,33 it does not seem to be
essential in mouse MK, where GPVI mediates the
inhibitory signal.34 Considering the key function of α2b1
in MK behavior over collagen, we next studied MK α2 sur-
face expression and found decreased levels on patient
mature (CD41+CD42+) MK (Figure 6A). There was a trend
towards reduced MK (CD41+) adhesion to fibrillar type I
collagen, which was confirmed after sorting the mature
++
(CD41 CD42 ) MK population, whereas adhesion to fib-
rinogen was preserved (Figure 6B and C). In addition to low α2 levels, other defects in FPD/AML MK could con- tribute to reduced collagen adhesion. Virtual absence of proplatelet formation from patient cells2 prevented us from assessing whether physiological collagen I-inhibition in thrombopoiesis was affected. The role of α2b1 in platelet production in vivo remains controversial as, unex- pectedly, Itga2-/- mice show normal platelet counts.32 Increased MK numbers found in these mice may represent a compensatory mechanism and provide an explanation for the absence of thrombocytopenia in this model. We have previously shown that FPD/AML patients show decreased MK output from hematopoietic progenitors,
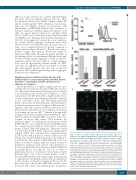
Figure 6. Levels of integrin subunit alpha (α)-2 and collagen adhesion in
megakaryocytes (MK) in familial platelet disorder with predisposition to acute
myelogenous leukemia (FPD/AML). (A) (Left) Flow cytometry analysis of integrin
CD34 cells from patients (n=3): DII-1 ( !), DIII-1 ( ), DIII-3 ( !), and healthy sub- jects (n=5). Median relative fluorescence intensity values and interquartile range are depicted; *P<0.05. (Right) Representative histograms of CD49b expression in patient (dashed line) and control (thick continuous black line) MK. Isotype controls in the patient and control are shown by superimposed solid gray and empty gray histograms, respectively. (B) MK adhesion to fibrillar type I colla- gen and fibrinogen in patients. The number of adherent cells was expressed as percentage of simultaneously assayed controls, set as 100%. (Left) Results for MK (CD41+) adhesion to collagen in patients (n=3: DII-1, DIII-1 and DIII-3). In sep- arate experiments, CD41+CD42+ cells were sorted by flow cytometry (n=2: DIII-3 and BIII-1), allowed to adhere to collagen or fibrinogen-coated surfaces; (middle and right) results are shown. Bars represent median values and interquartile range; P=not significant. (C) Representative images of patient and control adher- ent mature (CD41+CD42+) MK stained with phalloidin-fluorescein isothiocyanate (FITC) and 4′,6-diamidino-2-phenylindole.
α-2 expression in mature (CD41 CD42 ) MK grown from peripheral blood +++!
haematologica | 2019; 104(6)
1253
vs.