Page 95 - 2019_04-Haematologica-web
P. 95
New Drosophila model for chronic myeloid leukemia
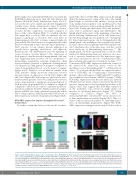
Interestingly, two independent RNAi lines worsened the BCR-ABL1 phenotype more than the Dab deletion did (Figures 2E and 5A, Online Supplementary Figure S2A,F-I): most of the eyes were smaller and showed depigmented scar-like tissue (Online Supplementary Figure S2A,F,H,I). Consistently, alterations of the ommatidia clusters, revealed by Elav expression, worsened compared to those of the control (Figure 5B-E). To establish whether Dab might have a role in CML we analyzed the two human counterparts of Disabled, Dab1 and Dab2 in human primary cells. Dab1 is a large, common fragile site gene and the Dab1 protein acts as a signal transducer that interacts with many receptor tyrosine kinase pathways.31 Dab2 encodes for an adaptor protein implicated in growth factor signaling, endocytosis, cell adhesion, hematopoietic cell differentiation and cell signaling of various receptor tyrosine kinases.32 The expression of both genes is often decreased in many human solid can- cers, suggesting their possible role in oncogenesis.31,33 Interestingly, quantitative real-time polymerase chain reaction analysis revealed a significant downregulation of both genes in CML patients at diagnosis compared to controls in peripheral blood or bone marrow samples (Figure 6A,G). Analysis of bone marrow samples from CML patients during molecular remission showed increased levels of expression of both Dab1 (Figure 6B) and Dab2 (Figure 6H) with respect to the levels in treat- ment-resistant patients. Moreover, immunofluorescence assays demonstrated a significant down-modulation of both proteins in peripheral blood samples at diagnosis compared to the levels in controls or patients in molecu- lar remission (Figure 6C,D,I,J). Finally, transfection exper- iments in K562 cells using a plasmid carrying the whole Dab1 coding sequence demonstrated that reactivation of Dab1 expression reduced cell proliferation (Figure 6E,F).
BCR-ABL1 expression impairs Drosophila blood cell homeostasis
To further confirm the efficacy of the model, we inves-
A
tigated the effects of BCR-ABL1 expression in the lymph gland, the hematopoietic organ of the larva. The lymph gland begins to develop in the embryo34 and grows up from multipotent progenitor cells (prohemocytes) that proliferate and enter a quiescent phase during the second instar (L2). During the third instar (L3) some prohemo- cytes start to proliferate again and differentiate. The lymph gland breaks apart at the beginning of metamor- phosis releasing differentiated blood cells (hemocytes) into the hemolymph, the Drosophila blood.35,36 During the L3, three functional regions can be distinguished in the lymph gland:37 the medullary zone, populated by prohe- mocytes; the posterior signaling center that regulates the exit of prohemocytes from quiescence; and the cortical zone, made up of differentiating hemocytes.38,39 The lymph gland can break up prematurely in late-L3 if the number of differentiating hemocyte increases. As a reac- tion to excessive hematopoiesis, the hemocytes aggre- gate and a spontaneous process of melanization takes place inducing the formation of melanotic nodules.11,36,40,41 Constitutive BCR-ABL1 expression under the control of the domelessGal4 (domeGal4) driver, active in the medullary zone of the lymph gland,11,42 is lethal (data not shown). To overcome this problem, we repressed expres- sion of BCR-ABL1 by co-expressing a heat-sensitive mutant of the Gal4 repressor Gal80 (tubGal80TS) until lar- vae reached the desired instar (TARGET system).10 While BCR-ABL1 expression from the first instar (L1) induced lethality (data not shown), expression from the L2 allowed larvae to survive and to develop melanotic nodules at L3 (Figure 7A,B). This suggests that BCR-ABL1 expression in the medullary zone precursors might induce an increase of circulating hemocytes (Figure 7C). When compared to controls (Figure 7A), 45% of domeGal4,BCR-ABL1 3M,tubGal80TS larvae showed two to three small melanotic nodules (Figure 7B,C). This cor- relates with an increased number of circulating hemo- cytes in hemolymph preparations (Figure 7D). BCR- ABL1 expression starting from the early L3 did not show
BC
DE
Figure 5. Dab downregulation enhances the eye phenotype due to BCR-ABL1. (A) Piled histogram chart showing the frequencies of the three phenotypic classes in flies co-expressing BCR-ABL1 (gmrGal4,4M) and EGFP (UAS-EGFP/+;gmrGal4,4M/+), or one of two independent Dab-RNAi constructs (VDRC#13005, VDRC#14008). (B,C) Eye imaginal discs from wild-type late third instar larvae expressing EGFP under the control of the gmrGal4 driver in cells posterior to the morphogenetic furrow and expressing the pan-neuronal marker Elav in cells committed to terminal differentiation. (D,E) Elav expression in eye imaginal discs from late third instar larvae expressing BCR-ABL1 (D) or larvae co-expressing BCR-ABL1 and Dab-RNAi (E) under the control of the gmrGal4 driver construct. BCR-ABL1 expression reduces the number of differentiated photoreceptors, as indicated by a decrease of Elav-expressing cells, and Dab downregulation enhances this phenotypic trait. The statistical comparisons were conducted using a Mann-Whitney test (*P<0.05, **P<0.01, ***P<0.001).
haematologica | 2019; 104(4)
723