Page 116 - 2019_01-Haematologica-web
P. 116
Y. Lu et al.
ABCD
E
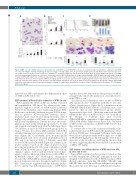
Figure 3. 2BP overcomes ATRA resistance in vitro and in vivo. (A-B) ATRA-resistant NB4-MR2 cells were treated with 5 mM or 10 mM 2BP in combination with ATRA for 3 days. The Wright’s staining morphology (A) and percentages of CD11b (upper panel, B) and CD11c (middle panel, B) and CD15 (bottom panel, B) expression are shown. Scale bars are 20 mm. The images were quantified as described in Figure 1G and shown at the bottom. *P<0.05 against ATRA-treated group. (C-D) ATRA- resistant transplantable leukemic mice (leu) were treated with vehicle, 2BP (5 mg per kg body weight, intraperitoneally), ATRA (10 mg per kg body weight, intraperi- toneally) or ATRA/2BP daily for five continuous days a week. The survival (%) and lifetime (day) of leukemic mice in each group were recorded and Kaplan–Meier sur- vival analysis is shown (C). *P<0.05 against ATRA-treated group. (D) The macroscopic appearance/the weight (mg/g bw) of spleen (top panels) are shown. Each col- umn represents the mean with bar as s.d. of 3 mice in an independent experiment, and *P<0.05 between the line-pointed group. (E) Cytologic analysis PB and BM cells derived from different agent-treated mice by Wright’s staining. The images were quantified as described in Figure 1G and shown on the bottom. *P<0.05 against ATRA-treated group.
indicated that 2BP could enhance the differentiation effect of ATO on APL cells in vitro.
2BP enhances differentiation induction of ATRA in vivo Subsequently, the effect of 2BP was further evaluated on transplantable APL mice. We intravenously trans- planted a high dose (4×105) of ATRA-sensitive leukemic blasts from transgenic mice expressing human PML- RARα into sublethally irradiated isogenic FVB/N recipi- ents to generate ATRA-sensitive leukemic mice.30 Leukemic mice were treated intraperitoneally with 5 mg/kg body weight of 2BP with or without ATRA on day 2 after transplantation. Twenty-five days after trans- plantation, mice in the vehicle group started to be frail and sluggish, and rapidly died off in the following 3 days. The lifetime of mice in ATRA-treated group lasted as long as 35 days. In contrast, 2BP combined with ATRA extended lifetime and survival of leukemic mice to 45 days (Figure 2A). Consistently, more morphologically differentiated cells were observed in peripheral blood (PB) and bone marrow (BM) of leukemic mice that received ATRA and ATRA/2BP (Figure 2B). Further quan- titative analysis based on the shape of nuclei showed more mature granulocytic cells upon combination of 2BP with ATRA (bottom panel, Figure 2B). In addition, enlarged spleens were found in vehicle-treated mice, which could be alleviated when treated with ATRA or ATRA/2BP (Figure 2C). Histological examination revealed that ATRA/2BP treatment inhibited the infiltra- tion of leukemia cells into the spleen and liver (Figure 2D). These results suggested that the leukemic infiltra- tion to spleen was reduced in presence of 2BP. Taken
together, these data demonstrated the potential of 2BP to synergistically induce the maturation of promyelocytes with ATRA in vivo.
In addition, we evaluated the effect of 2BP on ATO in APL murine model.31 Consistent with the in vitro data (Online Supplementary Figure S4A), administration of ATO/2BP extended the survival of leukemic mice com- pared to that in the ATO-treated group (Online Supplementary Figure S4B). These data supported that 2BP enhanced the effect of ATO on APL in vivo. In addition, the lifespan of leukemic mice that received ATRA/ATO/2BP is comparable to that when treated with ATRA/ATO (Online Supplementary Figure S4B). The effect of 2BP on the combination of ATRA/ATO deserves fur- ther evaluation.
To evaluate the in vivo acute toxicity and identify a clin- ically relevant dose of 2BP in mice, the maximum tolerat- ed dose (MTD) was determined.32 Athymic nude mice were injected intraperitoneally with a single dose of 2BP at 50,100,200 mg/kg/dose. At 100 mg/kg, treated mice were alive at day 14, whereas at 200 mg/kg, the mice died on day 2, suggesting that the best-tolerated concentration of 2BP is 100 mg/kg.
2BP induces the differentiation of ATRA-resistant APL in vitro and in vivo
Since ATRA resistance has been a major obstacle in clin- ical APL therapy, the ATRA/2BP combination was also designed to be examined to relieve ATRA resistance. NB4- derived subclones, including NB4-MR2, NB4-LR1 and NB4-LR2 were tested. In accordance with previous reports, these subclones were refractory to ATRA-induced matura-
106
haematologica | 2019; 104(1)