Page 61 - 2018_12-Haematologica-web
P. 61
Progressive hematopoietic defects in ASXL2 KO mice
ABC
DEFG
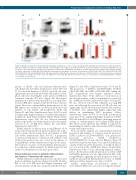
Figure 4. ASXL2 is essential for normal thymocyte maturation. (A) Thymocyte count in >1-year old wild-type (WT) and Asxl2 knockout (KO) mice. (B) Representative FACS plots depict the proportion of DN (CD4–CD8–), DP (CD4+CD8+), CD4+ single positive (SP) and CD8+ SP cells in the thymus of old WT and Asxl2 KO mice. (C) Frequencies of DP, DN, CD4+ SP and CD8+ SP populations in old WT (n=17) and Asxl2 KO (n=18) mice. (D) FACS plots show representative flow cytometric staining for surface expression of CD44 and CD25 within the DN population in the thymus of old mice. (E) Proportions of DN1 (CD44+CD25– DN), DN2 (CD44+CD25+ DN), DN3 (CD44–CD25+ DN) and DN4 (CD44–CD25– DN) sub-populations within the DN compartment of thymus of old WT and KO mice. (F and G) Frequencies of myeloid cells (CD11b+) (F) and B cells (CD19+) (G) in the thymus of >1-year old WT and KO mice (n=5-7). Data are represented as mean±Standard Error of Mean. ***P<0.001, ****P<0.0001.
quency of CD11b+ cells and myeloperoxidase-positive cells (Figure 2E and Online Supplementary Figure S8B and C). The absolute numbers of CD11b+ myeloid cells were significantly increased (20-fold) while the number of lym- phoid cells were 2-fold higher in the spleens of KO mice compared with the WT mice (Figure 2F). Histological examination of spleens demonstrated loss of normal archi- tecture in KO mice compared with the WT mice (data not shown). Moreover, extramedullary hematopoiesis in the KO spleens was evident by an elevated proportion and numbers of total Lin–Kit+ and Lin–Kit+Sca1+ (LSK) cells (Figure 2G and Online Supplementary Figure S9A-D) as well as significantly higher frequency of erythroid progenitors (populations proE, EryA and EryB) (Figure 2H and Online Supplementary Figure S9E). We also detected markedly increased frequency of stem/progenitor cells in the periph- eral blood of old KO mice (Figure 2G and Online Supplementary Figure S9F).
Parallel analyses of spleens from young KO mice (8-14 weeks old) revealed modestly increased spleen size with a trend towards elevated proportion and number of LSK cells (Online Supplementary Figure S10A-C). A propensity for increased frequencies of myeloid cells (CD11b+) and erythroid progenitors was also noted in the spleens of young KO mice (Online Supplementary Figure S10D and E). This indicates an onset of extramedullary hematopoiesis in young mice, which manifests by marked myeloid and erythroid cell expansion as the mice grow older.
ASXL2 deficiency results in defective differentiation and function of hematopoietic stem cells
ASXL2 deficiency resulted in paler bones and decreased marrow cellularity in old mice (Figure 3A and B). Notably, BM of old Asxl2 KO mice had higher frequency and absolute number of LSK cells compared with the WT mice
(Figure 3C and Online Supplementary Figure S11A and B). The proportion of LT-HSCs (CD34–Flt3–LSK), ST-HSCs (CD34+Flt3–LSK) and MPPs (CD34+Flt3+LSK) within the LSK compartment was largely unaltered (Online Supplementary Figure S11C), albeit an overall increase in the absolute number of these subpopulations occurred in the old KO mice (Online Supplementary Figure S11D and E). We also observed reduced BM cellularity in young KO mice; and although the proportion of LSK cells was not significantly altered, the frequency and absolute numbers of LT-HSCs were significantly higher, suggesting early defects in maintaining HSC frequency (Online Supplementary Figure S11F-J). In vivo BrdU incorporation assay uncovered a significantly higher frequency of BrdU+ LSK cells in the BM of Asxl2 KO mice, indicating increased cycling of stem/progenitor cells lacking ASXL2 (Figure 3D). These results illustrate that ASXL2 is required for maintaining the number and self-renewal of HSCs during steady-state hematopoiesis.
Flow cytometric analyses also demonstrated decreased proportion and number of common myeloid precursors (CMP; Lin–Kit+Sca1–CD34+FcgRII/IIIlo) and granulocyte monocyte precursors (GMP; Lin–Kit+Sca1– CD34+FcgRII/IIIhi) in >1-year old Asxl2 KO mice (Figure 3E and Online Supplementary Figure S12A and B). Furthermore, a marked reduction in the frequencies of erythroid precur- sors was noted in the old KO mice (Figure 3F and Online Supplementary Figure S12C), indicating impaired erythro- poiesis. Reduction in erythroid precursors as well as CMP and GMP populations was also apparent in the BM of the young KO mice, signifying an early inception of defects in erythroid and myeloid differentiation because of ASXL2 deficiency (Online Supplementary Figure S12D-F).
Further, multi-lineage reconstitution ability of Asxl2-deficient HSCs was assessed in a competitive repop-
haematologica | 2018; 103(12)
1985