Page 52 - 2018_12-Haematologica-web
P. 52
X. Liu et al.
prospective coronal suture mesenchyme and osteoprogen- itors.37,38 Moreover, aberrant Notch signaling is a common mechanism in niche-induced AML and pre-leukemic con- ditions.39-42 To investigate whether Twist1 deficiency pro- motes the development of MLL-AF9 AML through Notch signaling, we determined the expression of all Notch lig- ands (Dll1, Dll3, Dll4, Jagged-1, Jagged-2) in MSC, OLC and EC of Twist1-deleted mice. The results revealed that Jagged- 2 was significantly upregulated in all these cells (Figure 6B- D). Additionally, the levels of expression of all four Notch receptors (Notch1-4), cleaved Notch1 and the Notch targets Dtx, Hes1, Hes5, Hey1, and Hey2 were significantly upreg- ulated in LSC from Twist1-deleted mice compared to those of controls (Figure 6E-G), indicating increased Notch sig- naling in this population. Furthermore, pharmacological inhibition of Notch signaling with a g-secretase inhibitor (DBZ) (Figure 6H-K) or blockade of Notch with dominant- negative MAML1 (DNMAML1) (Online Supplementary Figure S7) partially rescued leukemic cell infiltration and LSC engraftment, and prolonged the overall survival of Twist1-deleted recipients. These data suggest that a Twist1- deleted microenvironment contributes to MLL-AF9 AML development at least in part via Notch signaling.
A
BC
Discussion
In the current study, we demonstrated that excision of the Twist1 gene from the BM microenvironment resulted in a significant decrease in the numbers of MSC and mature osteoblasts, and an increase in the number of EC. The expression of CXCL12, VCAM1 and SCF was reduced, while that of osteopontin was increased. These changes led to a marked impairment of HSC localization, self- renewal, quiescence and differentiation. By transplanting MLL-AF9 cells into the Twist1-deleted and control chimeric mice, we verified that Twist1 deletion resulted in accelerat- ed development of leukemia, at least partially through Notch signaling (Figure 7). These results reveal the essential role of TWIST1 in supporting normal hematopoiesis and perturbing AML development.
In our model, Twist1 deletion in the BM microenviron- ment leads to an increased number of EC and microvessel density, suggesting the existence of an indirect and power- ful mechanism for promoting angiogenesis in vivo. Ohki et al. reported that G-CSF can markedly increase vascular endothelial growth factor (VEGF) release from G-CSF- responsive myelomonocytic cells, which promote the co- recruitment of VEGFR1+ (VEGF receptor 1) cells contribut- ing to neo-angiogenesis.43 Since we have found elevated G-
DE
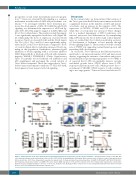
Figure 5. Twist1 deletion in the bone marrow microenvironment promotes the progression of acute myeloid leukemia. (A) Experimental scheme of the MLL-AF9 acute myeloid leukemia model and leukemic stem cell (LSC, GFP+c-Kit+Gr-1-) transplantation. (B) Kaplan–Meier survival curve of chimeric control (Ctrl) and knockout (KO) recipient mice (n=5, three independent experiments, log-rank test). (C) Representative flow cytometry profiles of L-GMP (IL-7R-Lin-GFP+c-KithiCD34+CD16/32hi). (D) Frequency and absolute number of L-GMP in the bone marrow (BM) and spleen of Ctrl and KO recipients (n=5, three independent experiments. Column plots show the mean ± standard deviation. **P<0.01; ***P<0.001, Student t test). (E) Kaplan–Meier survival curve of mice transplanted with LSC from chimeric Ctrl and KO mice (n=6, two independent experiments, log-rank test).
1976
haematologica | 2018; 103(12)