Page 62 - 2018_11-Haematologica-web
P. 62
C. Ka et al.
Discussion
The p.Arg178Gln missense mutation is typical of the fer- roportin 1 variants for which evidence remains inadequate and clinical pathogenicity doubtful. Herein, we provide a comprehensive genotype-phenotype analysis, and argue
that p.Arg178Gln substitution abolishes a salt bridge between the N and C lobes of human ferroportin 1, leading to a less stable outward facing state and, thus, to an altered equilibrium between the different conformational states.
The p.Arg178Gln missense mutation was first reported in a 70-year-old female with hyperferritinemia and normal
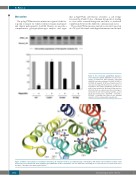
Figure 4. The ferroportin p.Arg178Gln mutant is not resistant to hepcidin. HEK293T cells were tran- siently co-transfected with plasmids expressing HLA(A)-V5 and either wild-type SLC40A1-V5 (WT) or SLC40A1-V5 variants. At 16h post-transfection, the cells were incubated in the presence or absence of hepcidin for 3h. Plasma membrane proteins were purified and analyzed by Western blotting and den- sitometry. Data are expressed as the percentage of ferroportin in cells not treated by hepcidin, accord- ing to the formula 100 x (SLC40A1 – hepcidin / SLC40A1 + hepcidin). Error bars are the standard deviation of three independent experiments.
TM8
TM1
N174 D473
TM10
TM7 TM12
TM5
R178 Q481
TM4
D157 R489
TM11
TM6
TM3
TM2
TM9
R88 E486
D84
Figure 5. Ribbon representation of a human ferroportin-1 3D structure model in an outward facing conformation, with atomic representation of amino acids involved in non-covalent bonds, likely leading to the stabilization of this conformation. The iron within the iron-binding site is represented as a red ball at the top of the figure. This figure was drawn using Chimera.38
1802
haematologica | 2018; 103(11)