Page 108 - 2018_10-Haematologica-web
P. 108
R. Jimenez-P. et al.
elevated in primary tumor samples derived from BL patients.
CDCA7 mediates anchorage-independent growth of lymphoma cells
The overexpression of CDCA7 in BL cells could be causally involved in oncogenesis or be only the result of a random event unrelated to tumor formation. To ascertain whether CDCA7 played a causative role in lymphomagen- esis, we knocked-down its expression in BL tumor cells by using lentivirus encoding CDCA7-specific shRNA. By screening candidate shRNAs specific for CDCA7 in lym- phoma cells, we identified sh-25 and sh-83 as having high knockdown capacity relative to a non-targeting shRNA (sh- Ctl) or to non-transduced cells (Online Supplementary Figure S5). Lentiviral transduction of DG-75 BL cells with sh-25 or sh-83 markedly inhibited CDCA7 expression relative to cells transduced with sh-Ctl (Figure 4A, left panel). CDCA7 silencing in these cells sharply decreased their colony for- mation capacity in soft agar (Figure 4B, left column; and Figure 4C, top panel). To determine whether CDCA7 plays a similar role in other BL cells, we transduced BL2 and Ramos BL cells with lentivirus encoding sh-25 or sh-83. These shRNAs efficiently silenced CDCA7 expression in these cells (Figure 4A, middle and right panels) and sharply decreased their growth in soft agar (Figure 4B, middle and right panels; and Figure 4C, middle and bottom panels).
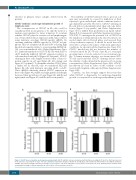
The inability of CDCA7-silenced cells to grow in soft agar may potentially be caused by inhibition of their capacity to grow under liquid culture conditions (anchor- age-dependent growth). However, CDCA7 silencing in BL cells did not substantially affect their cell cycle distri- bution [Figure 5 (top panels) and Online Supplementary Figure S6] or inhibit their proliferation in liquid culture [Figure 5 (bottom panels) and Online Supplementary Figures S7A-S7B]. To investigate the potential role of CDCA7 in the regulation of cell proliferation by cell-cell contacts, we seeded single cells in 96-well plates and assessed their colony formation capacity. It should be noted that cells were able to attach to the surface of the wells under these conditions. As shown in Online Supplementary Figure S7C, CDCA7 knockdown did not decrease the number of colonies formed by DG-75 cells. Since BL is a very rapid growing tumor, it can be subjected to poor nutrient sup- ply. To mimic nutrient shortage, we serum-starved DG- 75 cells and found that CDCA7 silencing did not affect the viability of cells cultured in the presence of low serum concentrations (Online Supplementary Figure S8A). CDCA7 knockdown also had no effect on the viability of cells treated with Cisplatin or Bleomycin (Online Supplementary Figure S8B).
Together, our data strongly support the notion that while CDCA7 is dispensable for anchorage-dependent growth, it is required for anchorage-independent growth
A
B
Figure 5. CDCA7 does not mediate anchorage-dependent growth. DG-75 and BL2 cells were transduced with lentivirus encoding sh-Ctl, sh-25 or sh-83 and selected in the presence of puromycin >5 days. Cell cycle and Edu incorporation analysis of (A) DG-75 and (B) BL2 cells transduced with lentivirus encoding the indicated shRNAs. (Top panels) Columns show the percentage of each of these cells in the indicated cell cycle phases as mean±s.e.m (n=3). (Bottom panels) Columns show normalized percentage of Edu incorporation in each of these cells as mean±s.e.m (n=3).
1674
haematologica | 2018; 103(10)