Page 59 - 2018_09-Mondo
P. 59
MΦ-mediated BM failure
A
B
CDEF
G
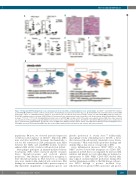
Figure 7. Podoplanin (PDPN) antagonism rescues hematopoietic stem cells (HSC), circulating platelet levels, and mortality. (A) CD41lo/int. and CD41hi HSC numbers in anti-PDPN (■; a-PDPN) or isotype control (□)-treated mice 8 and 14 days post-splenocyte transfer (d.p.s.t.). (B) Hematoxylin and eosin-stained bone marrow (BM) from isotype control- or a-PDPN-treated mice 14 d.p.s.t. Scale bar=50 μm. (C) Platelets in the blood of isotype control- (□) and α-PDPN (■)-treated mice 14 d.p.s.t. (D) GP1bβ+ megakaryocytes per 100mm2 of BM 14 d.p.s.t. Data represent one experiment repeated at least twice, n=4-18 mice/group. Mean±Standard Error of Mean is shown. **P<0.01, ***P<0.001. (E) Kaplan-Meier survival curve for a-PDPN- (■) or isotype control (□)-treated severe aplastic anemia (SAA) mice. Data represent one experiment, n=8 mice/group. Log-rank (Mantel-Cox) test was used to compare between groups. (F) CD11blo/- and CD11b+ MΦ populations were enumerated 8 d.p.s.t. (G) Schematic summarizing the steady state role(s) for MΦs in the BM (left) and the impact of IFNγ on the BM microenvironment and resulting HSC loss in SAA (right): increased PDPN-expressing MΦs that drive reduced Mks, impaired platelet production, and correlate with reduced stromal.
populations. Of note, we observed increased expression of M2-associated arginase1 in CD11blo/- Mfs from MIIG mice and after anti-PDPN treatment. Mechanistically, a role for M1 activation in SAA pathogenesis may differ between the MIIG and anti-PDPN models; however, enhanced M2 activity correlates with protection in SAA.
Macrophages participate in immune-mediated throm- bocytopenia (ITP), where increased platelet clearance drives thrombocytopenia and reduced platelet production.45 Mf clearance of platelets does not appear to drive thrombocytopenia in SAA, however, as clearance rates were similar in Mf−depleted and control mice. Our findings are consistent with and add to previous reports of Mf−dependent impairment of megakaryopoiesis and
platelet production at steady state.46,47 Additionally, macrophage-colony stimulating factor M-CSF, a factor that increases Mf self-renewal, transiently causes throm- bocytopenia.47 Our study builds upon these findings and identify Mfs as key sensors or target cells of IFNγ.
Acute inflammation increases CD41hi HSCs or SL- MkPs15 and we observed the emergence of a CD41hi HSC population in SAA that is accompanied by increased Mk numbers and platelet recovery when Mfs are depleted or unresponsive to IFNγ. While CD41hi HSC-derived Mks may support sustained platelet production during SAA, another intriguing possibility is that Mk-lineage cells pro- tect HSCs through a positive feedback loop. Mk-derived factors promote HSC quiescence and protect
haematologica | 2018; 103(9)
1459