Page 60 - Haematologica Vol. 107 - September 2022
P. 60
ARTICLE - Genetic abnormalities in older ALL patients
in only 13% (3/23) of cases with single variants in each of
DNMT3A, TET2 and ASXL1.
KDM6A alterations
Overall KDM6A was disrupted in six cases, with focal dele- tions in 5% (4/78) of SNP array samples (Table 3) and mu- tations in 9% (2/23) of NGS samples (Figure 3). Interestingly, the deletions resulted in homozygous or hemizygous KDM6A loss in three of the four cases (Figure 4A, Online Supplementary Table S7). Biallelic KDM6A dele- tions were seen in the two female patients with HoTr ALL, albeit by two different mechanisms. By cytogenetics and SNP array, patient #29407 had lost one copy of chromo- some X and had a focal KDM6A deletion in the remaining homologue. In comparison, patient #25437 had two focal but subtly distinct intragenic KDM6A microdeletions on each X chromosome (Figure 4B). As KDM6A is not in a
T. Creasey et al.
pseudoautosomal region, the male patient (#28011) had a deletion affecting the only KDM6A allele, resulting in hemizygous loss. The KDM6A mutations detected by NGS were present in exons 8 (KDM6A p.Y215H) and 20 (KDM6A p.K987Q) (Figure 5C) and are not reported in the literature although the SIFT31 and Polyphen32 in silico prediction tools describe deleterious and probably damaging con- sequences, respectively, consistent with loss of function. Patients with KDM6A deletions had a poor outcome and all four affected patients died 5-18 months after diagnosis (Table 2). Similarly, the two patients with KDM6A muta- tions both died within 2 months of diagnosis.
Patients’ outcome by genetic subtype
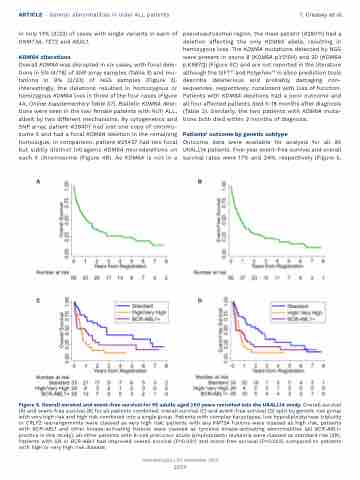
Outcome data were available for analysis for all 95 UKALL14 patients. Five-year event-free survival and overall survival rates were 17% and 24%, respectively (Figure 5,
AB
CD
Figure 5. Overall survival and event-free survival for 95 adults aged ≥60 years recruited into the UKALL14 study. Overall survival (A) and event-free survival (B) for all patients combined; overall survival (C) and event-free survival (D) split by genetic risk group with very high risk and high risk combined into a single group. Patients with complex karyotypes, low hypodiploidy/near triploidy or CRLF2 rearrangements were classed as very high risk; patients with any KMT2A fusions were classed as high risk; patients with BCR-ABL1 and other kinase-activating fusions were classed as tyrosine kinase-activating abnormalities (all BCR-ABL1- positive in this study); all other patients with B-cell precursor acute lymphoblastic leukemia were classed as standard risk (SR). Patients with SR or BCR-ABL1 had improved overall survival (P=0.001) and event-free survival (P=0.002) compared to patients with high or very high risk disease.
Haematologica | 107 September 2022
2059