Page 28 - Haematologica Vol. 107 - September 2022
P. 28
REVIEW ARTICLE - ITP: diagnosis and second-line treatment J.B. Bussel and C.A. Garcia
Haematologica | 107 September 2022
2027
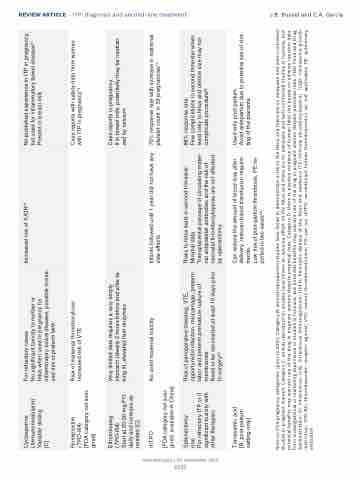
Cyclosporine (Immunomodulator) Variable dosing
[C]
For refractory cases
No significant toxicity to mother or
fetus when used in pregnancy for inflammatory bowel disease, possible increa- sed risk of preterm birth
Increased risk of IUGR70
No published experience in ITP in pregnancy, but used for inflammatory bowel disease91 Present in breast milk
Romiplostim
(TPO-RA)
[FDA category not assi- gned]
Risk of maternal thrombocytosis Increased risk of VTE
Case reports with safety data from women with ITP in pregnancy73
Eltrombopag (TPO-RA)
Start at 25-50 mg PO daily and increase as needed [C]
Very limited data requires a very empty stomach (ideally 2 hours before and after ta- king it), elevated liver enzymes
Case reports in pregnancy
If in breast milk, potentially may be neutrali- zed by calcium
rhTPO
No overt maternal toxicity
Infants followed until 1 year did not have any side effects
70% response rate with increase in maternal platelet count in 33 pregnancies72
[FDA category not assi- gned, available in China]
Splenectomy
(na)
For refractory ITP or if significant toxicity with other therapies
Risk of perioperative bleeding, VTE, opportunistic infection, miscarriage, preterm labor and preterm premature rupture of membranes
Need to be vaccinated at least 15 days prior to surgery53
Risks to fetus least in second trimester. Minimal data
Transplacental passage of circulating mater- nal antiplatelet antibodies and the risk of neonatal thrombocytopenia are not affected by splenectomy
88% response rate
Few complications in second trimester when least risky to fetus and uterine size may not complicate procedure92
Tranexamic acid [B, post-partum setting only]
Can reduce the amount of blood loss after delivery, reduces blood transfusion require- ments.
Low risk of post-partum thrombosis, PE re- ported in two cases62.
Used only post-partum.
Avoid antepartum due to potential risk of clot- ting of the placenta
Note on FDA pregnancy categories: (prior to 2015). Category B: animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women. Category C: animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks. Category D: there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks. FDA: Food and Drug Administration; IV: intravenous; IVIG: intravenous immunoglobulin; HDFN: hemolytic disease of the fetus and newborn; ITP: immune thrombocytopenia; IUGR: intrauterine growth restriction; TPO-RA: thrombopoietin receptor agonist; VTE: venous thromboembolism; PO: per os; rhTPO; recombinant human thrombopoietin; na: not applicable; PE: pulmonary embolism