Page 214 - Haematologica Vol. 107 - September 2022
P. 214
ARTICLE - Effects of specific inhibition of platelet MRP4 R. Wolf et al. A
B
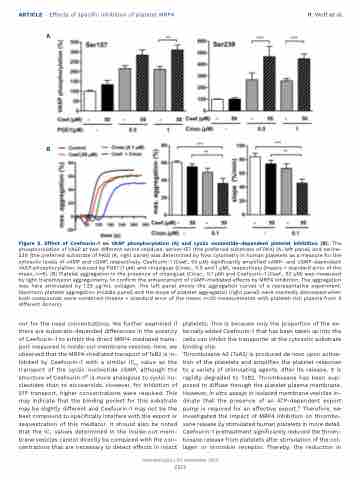
Figure 5. Effect of Ceefourin-1 on VASP phosphorylation (A) and cyclic nucleotide-dependent platelet inhibition (B). The phosphorylation of VASP at two different serine residues, serine-157 (the preferred substrate of PKA) (A, left panel) and serine- 239 (the preferred substrate of PKG) (A, right panel) was determined by flow cytometry in human platelets as a measure for the cytosolic levels of cAMP and cGMP, respectively. Ceefourin-1 (Ceef., 50 μM) significantly amplified cAMP- and cGMP-dependent VASP phosphorylation, induced by PGE1 (1 μM) and cinaciguat (Cinac., 0.5 and 1 μM), respectively (means + standard error of the mean, n=6). (B) Platelet aggregation in the presence of cinaciguat (Cinac., 0.1 μM) and Ceefourin-1 (Ceef., 50 μM) was measured by light transmission aggregometry, to confirm the enhancement of cGMP-mediated effects by MRP4 inhibition. The aggregation was here stimulated by 1.25 μg/mL collagen. The left panel shows the aggregation curves of a representative experiment. Maximum platelet aggregation (middle panel) and the slope of platelet aggregation (right panel) were markedly decreased when both compounds were combined (means + standard error of the mean; n=20 measurements with platelet-rich plasma from 4 different donors).
out for the used concentrations. We further examined if there are substrate-depended differences in the potency of Ceefourin-1 to inhibit the direct MRP4-mediated trans- port measured in inside-out membrane vesicles. Here, we observed that the MRP4-mediated transport of TxB2 is in- hibited by Ceefourin-1 with a similar IC50 value as the transport of the cyclic nucleotide cGMP, although the structure of Ceefourin-121 is more analogous to cyclic nu- cleotides than to eicosanoids. However, for inhibition of S1P transport, higher concentrations were required. This may indicate that the binding pocket for this substrate may be slightly different and Ceefourin-1 may not be the best compound to specifically interfere with the export or sequestration of this mediator. It should also be noted that the IC50 values determined in the inside-out mem- brane vesicles cannot directly be compared with the con- centrations that are necessary to detect effects in intact
platelets. This is because only the proportion of the ex- ternally added Ceefourin-1 that has been taken up into the cells can inhibit the transporter at the cytosolic substrate binding site.
Thromboxane A2 (TxA2) is produced de novo upon activa- tion of the platelets and amplifies the platelet response to a variety of stimulating agents. After its release, it is rapidly degraded to TxB2. Thromboxane has been sup- posed to diffuse through the platelet plasma membrane. However, in vitro assays in isolated membrane vesicles in- dicate that the presence of an ATP-dependent export pump is required for an effective export.13 Therefore, we investigated the impact of MRP4 inhibition on thrombo- xane release by stimulated human platelets in more detail. Ceefourin-1 pretreatment significantly reduced the throm- boxane release from platelets after stimulation of the col- lagen or thrombin receptor. Thereby, the reduction in
Haematologica | 107 September 2022
2213