Page 180 - Haematologica Vol. 107 - September 2022
P. 180
ARTICLE - Rituximab with LMB-chemotherapy regimen in PMLBL M.E. Dourthe et al.
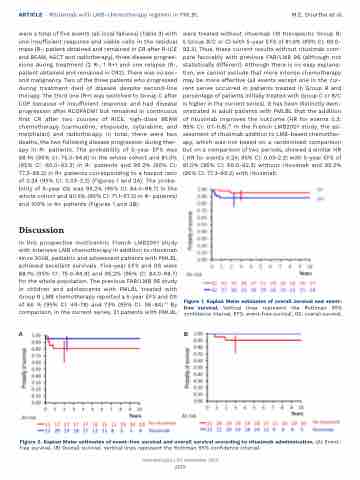
were a total of five events (all local failures) (Table 3) with one insufficient response and viable cells in the residual mass (R-, patient obtained and remained in CR after R-ICE and BEAM, ASCT and radiotherapy), three disease progres- sions during treatment (2 R-, 1 R+) and one relapse (R-, patient obtained and remained in CR2). There was no sec- ond malignancy. Two of the three patients who progressed during treatment died of disease despite second-line therapy. The third one (R+) was switched to Group C after COP because of insufficient response and had disease progression after RCOPADM1 but remained in continuous first CR after two courses of RICE, high-dose BEAM chemotherapy (carmustine, etoposide, cytarabine, and melphalan) and radiotherapy. In total, there were two deaths, the two following disease progression during ther- apy in R- patients. The probability of 5-year EFS was 88.1% (95% CI: 75.0-94.8) in the whole cohort and 81.0% (95% CI :60.0-92.3) in R- patients and 95.2% (95% CI: 77.3-99.2) in R+ patients corresponding to a hazard ratio of 0.24 (95% CI: 0.03-2.2) (Figures 1 and 2A). The proba- bility of 5-year OS was 95.2% (95% CI: 84.0-98.7) in the whole cohort and 90.5% (95% CI: 71.1-97.3) in R- patients) and 100% in R+ patients (Figures 1 and 2B).
Discussion
In this prospective multicentric French LMB2001 study with intensive LMB chemotherapy in addition to rituximab since 2008, pediatric and adolescent patients with PMLBL achieved excellent survivals. Five-year EFS and OS were 88.1% (95% CI: 75.0-94.8) and 95.2% (95% CI: 84.0-98.7) for the whole population. The previous FAB/LMB 96 study in children and adolescents with PMLBL treated with Group B LMB chemotherapy reported a 5-year EFS and OS of 66 % (95% CI: 49-78) and 73% (95% CI: 56-84).10 By comparison, in the current series, 21 patients with PMLBL
were treated without rituximab (16 therapeutic Group B; 5 Group B/C or C) with 5-year EFS of 81.0% (95% CI: 60.0- 92.3). Thus, these current results without rituximab com- pare favorably with previous FAB/LMB 96 (although not statistically different). Although there is no easy explana- tion, we cannot exclude that more intense chemotherapy may be more effective (all events except one in the cur- rent series occurred in patients treated in Group B and percentage of patients initially treated with Group C or B/C is higher in the current series). It has been distinctly dem- onstrated in adult patients with PMLBL that the addition of rituximab improves the outcome (HR for events 0.3; 95% CI: 0.1-0.8).16 In the French LMB2001 study, the as- sessment of rituximab addition to LMB-based chemother- apy, which was not based on a randomized comparison but on a comparison of two periods, showed a similar HR ( HR for events 0.24; 95% CI: 0.03-2.2) with 5-year EFS of 81.0% (95% CI: 60.0-92.3) without rituximab and 95.2% (95% CI: 77.3-99.2) with rituximab.
Figure 1. Kaplan Meier estimates of overall survival and event- free survival. Vertical lines represent the Rothman 95% confidence interval. EFS: event-free survival; OS: overall survival.
AB
Figure 2. Kaplan Meier estimates of event-free survival and overall survival according to rituximab administration. (A) Event- free survival. (B) Overall survival. Vertical lines represent the Rothman 95% confidence interval.
Haematologica | 107 September 2022
2179