Page 178 - Haematologica Vol. 107 - September 2022
P. 178
ARTICLE - Rituximab with LMB-chemotherapy regimen in PMLBL M.E. Dourthe et al.
sistent disease after the end of treatment received differ- ent therapies, including additional RT, second-line chemo- therapy, and consolidation with high-dose chemotherapy and autologous stem cell transplantation.
Statistical analysis
The primary efficacy endpoint was event-free survival (EFS), defined as the time from the start of chemotherapy to the first of the following events: biopsy-positive resid- ual disease following (R)CYVE number 2 or at the end of therapy, progressive disease, relapse, second malignant neoplasm, and death of any cause. Patients without any of these events were censored at the date of the last fol- low-up. The secondary efficacy endpoint was overall sur- vival (OS), defined as the time from the start of chemotherapy to death from any cause, or to the date of the last follow-up for alive patients. EFS and overall sur- vival (OS) were estimated with the Kaplan-Meier method.13 The 95% confidence intervals (95% CI) of the survival rates were calculated with the Rothman method.14
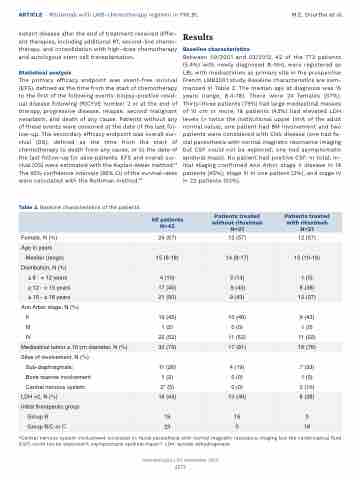
Table 2. Baseline characteristics of the patients.
Results
Baseline characteristics
Between 09/2001 and 03/2012, 42 of the 773 patients (5.4%) with newly diagnosed B-NHL were registered as LBL with mediastinum as primary site in the prospective French LMB2001 study. Baseline characteristics are sum- marized in Table 2. The median age at diagnosis was 15 years (range, 8.4-18). There were 24 females (57%). Thirty-three patients (79%) had large mediastinal masses of 10 cm or more, 18 patients (43%) had elevated LDH levels (> twice the institutional upper limit of the adult normal value), one patient had BM involvement and two patients were considered with CNS disease (one had fa- cial paresthesia with normal magnetic resonance imaging but CSF could not be explored; one had asymptomatic epidural mass). No patient had positive CSF. In total, in- itial staging confirmed Ann Arbor stage II disease in 19 patients (45%), stage III in one patient (2%), and stage IV in 22 patients (52%).
All patients N=42
Patients treated without rituximab N=21
Patients treated with rituximab N=21
Female, N (%)
24 (57)
12 (57)
12 (57)
Age in years
Median (range)
15 (8-18)
14 (8-17)
15 (10-18)
Distribution, N (%)
≥ 8 - < 12 years
4 (10)
3 (14)
1 (5)
≥ 12 - < 15 years
17 (40)
9 (43)
8 (38)
≥ 15 - ≤ 18 years
21 (50)
9 (43)
12 (57)
Ann Arbor stage, N (%)
II
19 (45)
10 (48)
9 (43)
III
1 (2)
0 (0)
1 (5)
IV
22 (52)
11 (52)
11 (52)
Mediastinal tumor ≥ 10 cm diameter, N (%)
33 (79)
17 (81)
16 (76)
Sites of involvement, N (%)
Sub-diaphragmatic
11 (26)
4 (19)
7 (33)
Bone marrow involvement
1 (2)
0 (0)
1 (5)
Central nervous system
2* (5)
0 (0)
2 (10)
LDH >2, N (%)
18 (43)
10 (48)
8 (38)
Initial therapeutic group
Group B
19
16
3
Group B/C or C
23
5
18
*Central nervous system involvement consisted in: facial paresthesia with normal magnetic resonance imaging but the cerebrospinal fluid (CSF) could not be explored=1; asymptomatic epidural mass=1. LDH: lactate dehydrogenase.
Haematologica | 107 September 2022
2177