Page 128 - Haematologica Vol. 107 - September 2022
P. 128
ARTICLE - Effects of BT200 on VWF/FVIII in humans
No apparent sex differences were noted in pharmacoki-
netic or pharmacodynamic values.
Desmopressin challenge
Eight subjects were included in part C, of whom four (in- cluding 1 woman) were re-exposed from part A. All were Caucasian and their mean age was 38 years (SD 13) (On- line Supplementary Table S4). Desmopressin did not alter BT200 exposure in any meaningful manner (data not shown). In fact, the responses to BT200 and desmo- pressin appeared to be additive, with the higher FVIII ac- tivity observed following BT200 plus desmopressin infusion taking a proportionally longer time to return to baseline (Figure 6).
Half-life estimation
To estimate the increase in half-life of endogenous VWF/FVIII the following assumptions were made: (i) 4-fold higher levels of VWF/FVIII will increase the AUC 4-fold; (ii) BT200 does not alter endogenous VWF/FVIII release/pro- duction (as evidenced by unchanging VWF propeptide levels); (iii) endogenous VWF/FVIII release/production is constant (i.e., similar to a continuous infusion of VWF/FVIII at a constant dose); and (iv) the volume of distribution of VWF does not change (as BT200 is a large molecule with predominantly intravascular distribution).
K.D. Kovacevic et al.
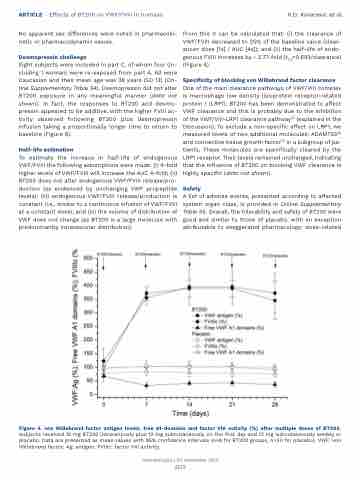
From this it can be calculated that: (i) the clearance of VWF/FVIII decreased to 25% of the baseline value (clear- ance= dose [1x] / AUC [4x]); and (ii) the half-life of endo- genous FVIII increases by ~ 2.77-fold (t1/2=0.693/clearance) (Figure 4).
Specificity of blocking von Willebrand factor clearance
One of the main clearance pathways of VWF/VIII complex is macrophage low density lipoprotein receptor-related protein 1 (LRP1). BT200 has been demonstrated to affect VWF clearance and this is probably due to the inhibition of the VWF/VIII-LRP1 clearance pathway23 (explained in the Discussion). To exclude a non-specific effect on LRP1, we measured levels of two additional molecules: ADAMTS524 and connective tissue growth factor25 in a subgroup of pa- tients. These molecules are specifically cleared by the LRP1 receptor. Their levels remained unchanged, indicating that the influence of BT200 on blocking VWF clearance is highly specific (data not shown).
Safety
A list of adverse events, presented according to affected system organ class, is provided in Online Supplementary Table S5. Overall, the tolerability and safety of BT200 were good and similar to those of placebo, with an exception attributable to exaggerated pharmacology: dose-related
Figure 4. von Willebrand factor antigen levels, free A1-domains and factor VIII activity (%) after multiple doses of BT200.
Subjects received 12 mg BT200 intravenously plus 12 mg subcutaneously on the first day and 12 mg subcutaneously weekly or placebo. Data are presented as mean values with 95% confidence intervals (n=6 for BT200 groups, n=20 for placebo). VWF: von Willebrand factor; Ag: antigen; FVIIIc: factor VIII activity.
Haematologica | 107 September 2022
2127