Page 35 - Haematologica May 2022
P. 35
lncRNA variants in CN-AML
ABC
DEF
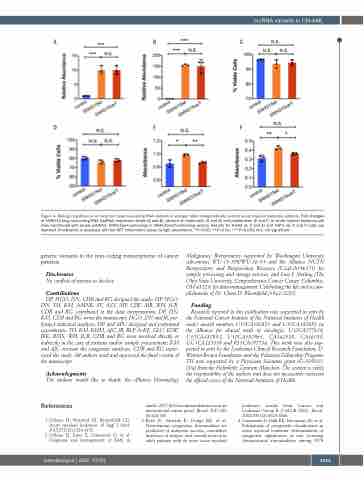
Figure 4. Biologic significance of recurrent long non-coding RNA variants in younger adult cytogenetically normal acute myeloid leukemia patients. Fold changes of SNHG15 long non-coding RNA (lncRNA) expression levels (A and B), percent of viable cells (C and D) and proliferation (E and F) of acute myeloid leukemia cell lines transfected with empty pcDNA3, SNHG15wt-containing or SNHG15varT-containing vectors. Results for K-562 (A, C and E) and THP-1 (B, D and F) cells are depicted. Proliferation is assessed with the MTT colorimetric assay, by light absorbance. *P<0.05; **P<0.01; ***P<0.001; N.S: not significant.
genetic variants in the non-coding transcriptome of cancer patients.
Disclosures
No conflicts of interest to disclose.
Contributions
DP, HGO, DN, CDB and RG designed the study; DP, HGO, DN, TH, KM, AMNB, SV, ASY, MP, CJW, MB, WH, JCB, CDB and RG contributed to the data interpretation; DP, DN, KM, CDB and RG wrote the manuscript; HGO, DN and JK per- formed statistical analysis; DP and APU designed and performed experiments; TH, KM, KHM, AJC, JB, BLP, A-KE, GLU, ESW, JEK, RMS, WH, JCB, CDB and RG were involved directly or indirectly in the care of patients and/or sample procurement; KM and AJC oversaw the cytogenetic analyses. CDB and RG super- vised the study. All authors read and approved the final version of the manuscript.
Acknowlegments
The authors would like to thank: the Alliance Hematology
Malignancy Biorepository supported by Washington University subcontract WU-15-398/WU-16-51 and the Alliance NCTN Biorepository and Biospecimen Resource (U24CA196171) for sample processing and storage services, and Lisa J. Sterling (The Ohio State University, Comprehensive Cancer Center, Columbus, OH 43221) for data management. Celebrating the life and accom- plishments of Dr. Clara D. Bloomfield (1942-2020).
Funding
Research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821 and U10CA180882 (to the Alliance for clinical trials in oncology), U10CA077658, U10CA180850, U10CA180861, CA140158, CA16058, UG1CA233338 and R35CA197734. This work was also sup- ported in part by the Leukemia Clinical Research Foundation, D. Warren Brown Foundation and the Pelotonia Fellowship Program. TH was supported by a Physician Scientists grant (G-509200- 004) from the Helmholtz Zentrum München. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136-1152.
2. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in
adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129 (4):424-447.
3. Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid
leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100 (13):4325-4336.
4. Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876
haematologica | 2022; 107(5)
1043