Page 211 - Haematologica May 2022
P. 211
CASE REPORTS
Platelet-activating anti-PF4 antibodies mimic VITT anti- bodies in an unvaccinated patient with monoclonal gammopathy
Transient prothrombotic disorders caused by platelet- activating antibodies against platelet factor 4 (PF4) include heparin-induced thrombocytopenia (HIT), spontaneous HIT syndrome,1 and, most recently, vaccine-induced immune thrombotic thrombocytopenia (VITT).2 Here, we identified prothrombotic, platelet-activating anti-PF4 anti- bodies, not associated with heparin treatment, in a patient with monoclonal gammopathy that resulted in a chronic hypercoagulability state.
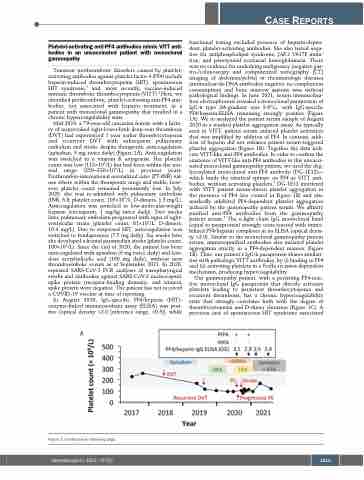
Mid-2019, a 79-year-old caucasian female with a histo- ry of unprovoked right-lower-limb deep-vein thrombosis (DVT) had experienced 1 year earlier thrombocytopenia and recurrent DVT with subsequent pulmonary embolism and stroke despite therapeutic anticoagulation (apixaban, 5 mg twice daily) (Figure 1A). Anticoagulation was switched to a vitamin K antagonist. Her platelet count was low (115×109/L) but had been within the nor- mal range (250–330×109/L) in previous years. Prothrombin-international normalized ratio (PT-INR) val- ues where within the therapeutic range and stable; how- ever platelet count remained persistently low. In July 2020, she was re-admitted with pulmonary embolism (INR, 3.8; platelet count, 105×109/L; D-dimers, 1.5 mg/L). Anticoagulation was switched to low-molecular-weight heparin (enoxaparin, 1 mg/kg twice daily). Two weeks later, pulmonary embolism progressed with signs of right- ventricular strain (platelet count, 81×109/L; D-dimers, 10.4 mg/L). Due to suspected HIT, anticoagulation was switched to fondaparinux (7.5 mg daily). Six weeks later she developed a frontal paramedian stroke (platelet count, 100×109/L). Since the end of 2020, the patient has been anticoagulated with apixaban (5 mg twice daily) and low- dose acetylsalicylic acid (100 mg daily), without new thromboembolic events as of September 2021. In 2020, repeated SARS-CoV-2 PCR analyses of nasopharyngeal swabs and antibodies against SARS-CoV-2 nucleocapsid, spike protein (receptor-binding domain), and trimeric spike protein were negative. The patient has not received a COVID-19 vaccine at time of reporting.
In August 2020, IgG-specific PF4/heparin (HIT)- enzyme-linked immunosorbant assay (ELISA) was posi- tive (optical density >2.0 [reference range, <0.5]), while
functional testing excluded presence of heparin-depen- dent, platelet-activating antibodies. She also tested nega- tive for antiphospholipid syndrome, JAK2 V617F muta- tion, and paroxysmal nocturnal hemoglobinuria. There was no evidence for underlying malignancy (negative gas- tro-/colonoscopy and computerized tomography [CT] imaging of abdomen/pelvis) or rheumatologic diseases (antinuclear/ds-DNA antibodies negative, no complement consumption) and bone marrow aspirate was without pathological findings. In June 2021, serum immunofixa- tion electrophoresis revealed a monoclonal paraprotein of IgG-κ type (M-gradient was 9.6%), with IgG-specific PF4/heparin-ELISA remaining strongly positive (Figure 1A). We re-analyzed the patient serum sample of August 2020 in a washed platelet aggregation assay. As typically seen in VITT, patient serum induced platelet activation that was amplified by addition of PF4. In contrast, addi- tion of heparin did not enhance patient serum-triggered platelet aggregation (Figure 1B). Together the data indi- cate VITT-like anti-PF4 antibodies. In order to confirm the existence of VITT-like anti-PF4 antibodies in this unvacci- nated monoclonal gammopathy patient, we used the deg- lycosylated monoclonal anti-PF4 antibody (DG-1E12)— which binds the identical epitope on PF4 as VITT anti- bodies, without activating platelets.3 DG-1E12 interfered with VITT patient serum-driven platelet aggregation in the presence of PF4 (see control in Figure 1B) and also markedly inhibited PF4-dependent platelet aggregation induced by the gammopathy patient serum. We affinity purified anti-PF4 antibodies from the gammopathy patient serum.4 The k-light chain IgG monoclonal band (equal to paraprotein) strongly cross-reacted with immo- bilized PF4/heparin complexes in an ELISA (optical densi- ty >2.0). Similar to the monoclonal gammopathy patient serum, immunopurified antibodies also initiated platelet aggregation strictly in a PF4-dependent manner (Figure 1B). Thus, our patient’s IgG-k paraprotein shares similari- ties with pathologic VITT antibodies, by (i) binding to PF4 and (ii) activating platelets in a FcgIIa receptor-dependent mechanism, producing hypercoagulability.
Our gammopathy patient, with a persisting PF4-reac- tive monoclonal IgG paraprotein that directly activates platelets leading to persistent thrombocytopenia and recurrent thrombosis, has a chronic hypercoagulability state that strongly correlates both with the degree of thrombocytopenia and D-dimer elevation (Figure 1C). A previous case of spontaneous HIT syndrome associated
A
Figure 1. Continued on following page.
haematologica | 2022; 107(5)
1219