Page 197 - Haematologica May 2022
P. 197
Letters to the Editor
Outstanding outcomes in infants with KMT2A- germline acute lymphoblastic leukemia treated with chemotherapy alone: results of the Children’s Oncology Group AALL0631 trial
Among infants with acute lymphoblastic leukemia (ALL), approximately 70-75% have KMT2A rearrange- ment (KMT2A-r) and 25-30% have KMT2A-germline (KMT2A-g) leukemia. Event-free (EFS) and overall sur- vival (OS) for KMT2A-g infant ALL are significantly bet- ter than those of KMT2A-r infant ALL, but inferior to out- comes in older children with ALL. Aside from the absence of KMT2A-r, the well-defined prognostic factors in older children with B-ALL (age, initial white blood cell [WBC] count, cytogenetics) are not clearly established, as KMT2A-g infant ALL accounts for only ~1% of child- hood ALL. Pediatric cooperative group trials conducted between 1996 and 2016 shown that the 4-6-year EFS/OS for infants with KMT2A-g ALL have ranged from 60-74% and 75-87%, respectively.1-4 Although the Japanese Infant Leukemia Study Group and the Japanese Pediatric Leukemia/Lymphoma Study Group reported remarkable 5-year EFS of 96% and 93% in KMT2A-g infant ALL in MLL96/98 and MLL-10, respectively, the cohort sizes were small, the sex ratios were skewed and the results have not been replicated in other cooperative groups.5-7 Children’s Oncology Group (COG) AALL0631 (clinical-
trials gov. Identifier: NCT00557193) was a phase III clin- ical trial for infants with newly diagnosed ALL with or without KMT2A-r.8 The trial was approved by Institutional Review Boards at participating COG mem- ber institutions and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from the parents or guardians according to federal and local regulations. The primary aim of AALL0631 was to test the safety and efficacy of the addition of the FLT3 inhibitor, lestaurtinib, to chemotherapy for infants with KMT2A-r.8 Infants with KMT2A-g were treated on a chemotherapy only arm, with a modified Interfant-99 based induction3,9 followed by modified COG P94079 therapy, with continuation therapy extending to 2 years from diagnosis (as compared to 46 weeks in the predeces- sor P9407 trial). The trial enrolled 64 infants with KMT2A-g and resulted in survival outcomes superior to those reported for infants with KMT2A-g in all prior COG and Interfant trials.
Infants less than 366 days of age with a new diagnosis of B- or T-cell lineage ALL, acute undifferentiated leukemia (AUL), or mixed phenotype acute leukemia (MPAL) with predominantly lymphoid morphology and immunophenotype were eligible to enroll. Neonates less than 4 weeks old and at least 36 weeks gestational age at the time of diagnosis were eligible. Patients with Down syndrome, mature B-cell leukemia, acute myeloid
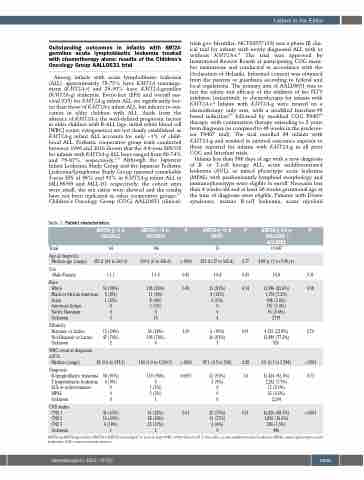
Table 1. Patient characteristics. KMT2A-g <1 yr
AALL0631
1:1.1 1:1.4
53 (90%) 108 (83%) 5 (8%) 11 (8%) 1 (2%) 8 (6%)
0 3 (2%) 0 0
5 16
15 (24%) 34 (24%) 47 (76%) 106 (76%) 2 6
38 (0.6 to 918.2) 160 (1.6 to 4,334.5)
KMT2A-r <1 yr AALL0631
P
KMT2A-g <1 yr P9407
P
0.77 0.03 0.34
0.61
0.28 1.0
20 (57%) 0.51 13 (37%)
KMT2A-g 1-9 yr P AALL03B1 +
AALL08B1
19,047 4.08yr(1to9.98yr)
1:0.8 0.31
13,986 (85.6%) 0.58 1,174 (7.2%)
908 (5.6%) 167 (1.0%) 93 (0.6%) 2719
4,132 (22.8%) 0.76 13,989 (77.2%)
146
Medianage(range) 281d(54to363d) 169d(0to360d) <.0001 291d(17to365d)
Total 64
35
1:0.4
25 (81%) 4 (13%) 2 (6%) 0
0
4
6 (19%) 26 (81%) 3
87.1 (2.5 to 540)
Age at diagnosis
Sex Male:Female
Race
White
Black or African American Asian
American Indian
Native Hawaiian Unknown
Ethnicity
Hispanic or Latino Not Hispanic or Latino Unknown
WBC count at diagnosis, x109/L
Median (range)
0.45 0.45
1.00
010
926
9.9 (0.1 to 5,784)
<.0001
Diagnosis
B-lymphoblastic leukemia 58 (91%) 139 (96%)
T-lymphoblastic leukemia 6 (9%) 0
AUL or indeterminate 0 1 (1%)
MPAL 0 5(3%) 0 32(0.2%)
Unknown
CNS status CNS 1 CNS 2 CNS 3 Unknown
15 (0.1%) 2,294
<.0001 0.0007
32 (91%) 3 (9%) 0
15,424 (92.1%) 0.73 1,282 (7.7%)
41 (65%) 16 (25%) 6 (10%)
61 (42%) 0.01 58 (40%)
25 (17%)
16,426 (88.3%) 1,895 (10.2%) 280 (1.5%)
<.0001
2 (6%)
1 2 0 446
KMT2A-g,KMT2A-germline;KMT2A-r:KMT2A-rearranged;yr:year;d:days;WBC:white blood cell;L:liter;AUL:acute undifferentiated leukemia;MPAL:mixed phenotype acute leukemia; CNS: central nervous system.
haematologica | 2022; 107(5)
1205