Page 195 - Haematologica May 2022
P. 195
Letters to the Editor
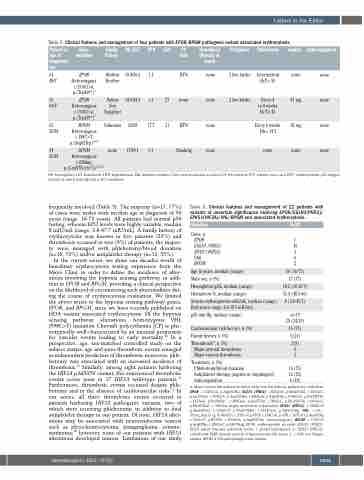
Table 2. Clinical features and management of four patients with EPOR/BPGM pathogenic variant associated erythrocytosis.
Patient n/ age at diagnosis/ sex
#1 48/F
Gene mutation
EPOR
Heterozygous c.1316G>A, p.(Trp439*)9
Family history
Hb/HcT
EPO
p50
CV Thrombosis risks (therapy at
event)
HTN none
Pregnancy
2 live births
2 live births
Phlebotomy
Intermittent HcT< 50
Every 4 to 8 weeks HcT< 43
Every 6 weeks Hb< 14.5
none
Aspirin
Anticoagulation
Mother 19.4/56.6 1.1 Brother
none none
81 mg none
81 mg none
none none
#2 EPOR Father 14.6/44.3 <1 27 none none 69/F Heterozygous Son
c.1316G>A, Daughter p.(Trp439*)9
#3 BPGM Unknown 20/58 17.7 31 HTN none 25/M Heterozygous
c.184C>T, p.(Arg62Trp)10,11
#4 BPGM none 17/49.1 5.1 Smoking none 25/M Heterozygous
c.258dup, p.(Leu87Serfs*3)10,12,13
Hb: hemoglobin; HcT: hematocrit; HTN: hypertension; DM: diabetes mellitus; CVA: cerebrovascular accident; LV: left ventricle; IVC: inferior vena cava; EPO: erythropoietin; p50: oxygen tension at which hemoglobin is 50% saturated.
frequently involved (Table 3). The majority (n=17, 77%) of cases were males with median age at diagnosis of 50 years (range, 16-73 years). All patients had normal p50 testing, whereas EPO levels were highly variable, median 8 mIU/mL (range, 3.8-47.7 mIU/mL). A family history of erythrocytosis was known in five patients (23%) and thrombosis occurred in two (9%) of patients; the majori- ty were managed with phlebotomy/blood donation (n=16, 73%) and/or antiplatelet therapy (n=12, 55%).
In the current series, we share our decades worth of hereditary erythrocytosis testing experience from the Mayo Clinic in order to define the incidence of alter- ations involving the hypoxia sensing pathway, in addi- tion to EPOR and BPGM, providing a clinical perspective on the likelihood of encountering such abnormalities dur- ing the course of erythrocytosis evaluation. We limited the above series to the hypoxia sensing pathway genes, EPOR, and BPGM, since we have recently published on HOA variant associated erythrocytosis. Of the hypoxia sensing pathway alterations, homozygous VHL (598C>T) mutation Chuvash polycythemia [CP] is phe- notypically well-characterized by an unusual propensity for vascular events leading to early mortality.14 In a prospective, age, sex-matched controlled study on the subject matter, age and prior thrombotic events emerged as independent predictors of thrombosis; moreover, phle- botomy was associated with an increased incidence of thrombosis.15 Similarly, among eight patients harboring the HIF2A p.M535V variant, five experienced thrombotic events versus none in 17 HIF2A wild-type patients.15 Furthermore, thrombotic events occurred despite phle- botomy and in the absence of cardiovascular risks.15 In our series, all three thrombotic events occurred in patients harboring HIF2A pathogenic variants, two of which were receiving phlebotomy, in addition to dual antiplatelet therapy in one patient. Of note, HIF2A alter- ations may be associated with neuroendocrine tumors such as pheochromocytoma, paraganglioma, somato- statinoma;16 however, none of our patients with HIF2A alterations developed tumors. Limitations of our study
Table 3. Clinical features and management of 22 patients with variants of uncertain significance involving EPOR/EGLN1(PHD2)/ EPAS1(HIF2A)/VHL/BPGM and associated erythrocytosis.
Variables
Gene, n
EPOR
EGLN1 (PHD2) EPAS1 (HIF2A) VHL
BPGM
Age in years, median (range)
Male sex, n (%)
Hemoglobin g/dL, median (range)
Hematocrit %, median (range)
Serum erythropoietin mIU/mL, median (range) Reference range, 2.6-18.5 mIU/mL
p50 mm Hg, median (range)
Cardiovascular risk factors, n (%)
Family history, n (%)
Thrombosisa, n (%)
Major arterial thrombosis Major venous thrombosis
Treatment, n (%)
Phlebotomy/blood donation
Antiplatelet therapy (aspirin or clopidogrel) Anticoagulation
N=22
1 10 3 6 2
50 (16-73) 17 (77) 18.2 (16-20.7) 53.4 (48.5-60) 8 (3.8-47.7)
n=19 25 (24-29)
16 (73) 5(23) 2(9)
0 2
16 (73) 12 (55) 4 (18)
a. Major venous thrombosis included deep vein thrombosis,pulmonary embolism. EPOR- c.1310G>A, p.Arg437His, EGLN1 (PHD2)- c.826A>G, p.Met276Val, c.165G>C, p.Lys55Asn, c.709G>C, p.Asp237His, c.280A>G, p.Arg94Gly, c.1016G>C, p.Ser339Thr, c.112A>G, p.Ser38Gly , c.289G>A, p.Ala97Thr, c.586G>C, p.Glu196Gln, c.604A>G, p.Met202Val, c.*92G>A, single nucleotide substitution, EPAS1 (HIF2A)- c.1958C>T, p.Ala653Val, c.1556C>T, p.Thr519Met, c.1834G>A, p.Gly612Arg VHL- c.-61_- 51het_dup11 (g.10183420), c.241C>T, p.P81S, c.134C>G ,p.45R, c.167C>G, p.Ala56Gly, c.345C>T, p.H155H, c.599G>A, p.Arg200Gln (heterozygous), BPGM- c.115C>T, p.Arg39Trp, c.289G>C, p.Gly97Arg. EPOR: erythropoietin receptor; EGLN1 (PHD2): EGL-9 family hypoxia inducible factor 1 (prolyl hydroxylase 2); EPAS1 (HIF2A): endothelial PASS domain protein 1(hypoxia-inducible factor 2 ); VHL: von Hippel Lindau; BPGM: 2,3-bisphosphoglycerate mutase.
haematologica | 2022; 107(5)
1203