Page 183 - Haematologica May 2022
P. 183
Letters to the Editor
(P=0.3) or the Moffitt Cancer Center cohort (P=0.7). In addition, there were no differences in OS between patients with mutations in the K700E hotspot and non- K700E sites in either the Mayo Clinic cohort (median OS 49 [95% CI: 22-109] months vs. 67 [95% CI: 36-126] months, P=0.5) (Online Supplementary Figure S1A) or the Moffitt Cancer Center cohort (median OS 85 [95% CI) months vs. not reached, P=0.9) (Online Supplementary Figure S1B).
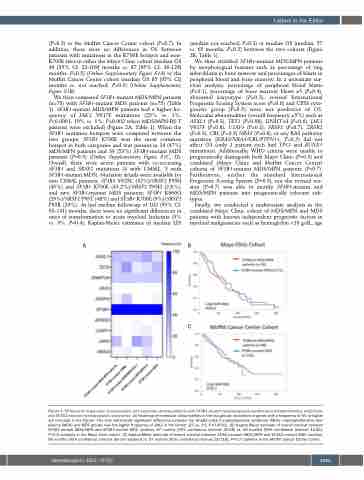
We then compared SF3B1-mutant MDS/MPN patients (n=78) with SF3B1-mutant MDS patients (n=75) (Table 1). SF3B1-mutant MDS/MPN patients had a higher fre- quency of JAK2 V617F mutations (25% vs. 1%, P<0.0001; 10% vs. 1%, P=0.002 when MDS/MPN-RS-T patients were excluded) (Figure 2A, Table 1). When the SF3B1 mutation hotspots were compared between the two groups, SF3B1 K700E was the most common hotspot in both categories and was present in 24 (47%) MDS/MPN patients and 39 (53%) SF3B1-mutant MDS patients (P=0.5) (Online Supplementary Figure S1C, D). Overall, there were seven patients with co-occurring SF3B1 and SRSF2 mutations (4 with CMML, 3 with SF3B1-mutant MDS). Mutation details were available for two CMML patients; SF3B1 Y623C (42%)/SRSF2 P95H (45%) and SF3B1 K700E (45.2%)/SRSF2 P95H (2.8%), and two SF3B1-mutant MDS patients; SF3B1 K666Q (29%)/SRSF2 P95T (48%) and SF3B1 K700E (9%)/SRSF2 P95R (29%). At last median follow-up of 102 (95% CI: 63-141) months, there were no significant differences in rates of transformation to acute myeloid leukemia (5% vs. 3%, P=0.4), Kaplan-Meier estimates of median LFS
(median not reached, P=0.3) or median OS (median, 57 vs. 65 months, P=0.2) between the two cohorts (Figure 2B, Table 1).
We then stratified SF3B1-mutant MDS/MPN patients by morphological features such as percentage of ring sideroblasts in bone marrow and percentages of blasts in peripheral blood and bone marrow. In a univariate sur- vival analysis, percentage of peripheral blood blasts (P=0.1), percentage of bone marrow blasts ≥5 (P=0.4), abnormal karyotype (P=0.3), revised International Prognostic Scoring System score (P=0.8) and CPSS cyto- genetic group (P=0.5) were not predictive of OS. Molecular abnormalities (overall frequency ≥5%) such as ASXL1 (P=0.3), TET2 (P=0.08), DNMT3A (P=0.6), JAK2 V617F (P=0.8), U2AF1 (P=0.2), SRSF2 (P=0.7), ZRSR2 (P=0.3), CBL (P=0.3) NRAS (P=0.8), or any RAS pathway mutation (KRAS/NRAS/CBL/PTPN11, P=0.3) did not affect OS (only 1 patient each had TP53 and RUNX1 mutations). Additionally, WHO criteria were unable to prognostically distinguish both Mayo Clinic (P=0.3) and combined (Mayo Clinic and Moffitt Cancer Center) cohorts of SF3B1-mutant MDS/MPN patients (P=0.7). Furthermore, neither the standard International Prognostic Scoring System (P=0.3), nor the revised ver- sion (P=0.7) was able to stratify SF3B1-mutant and MDS/MPN patients into prognostically relevant sub- types.
Finally, we conducted a multivariate analysis in the combined Mayo Clinic cohort of MDS/MPN and MDS patients with known independent prognostic factors in myeloid malignancies such as hemoglobin <10 g/dL, age
AB
C
Figure 2. Differences in genomic characteristics and outcomes among patients with SF3B1-mutant myelodysplastic syndrome/myeloproliferative neoplasms and SF3B1-mutant myelodysplastic syndromes. (A) Heatmap of molecular abnormalities in the two groups (mutations in genes with a frequency of 5% or higher are included in the figure). The only statistically significant difference between the SF3B1-mutant myelodysplastic syndrome (MDS) /myeloproliferative neo- plasms (MPN) and MDS groups was the higher frequency of JAK2 in the former (25 vs. 1%, P<0.0001). (B) Kaplan-Meier estimate of overall survival between SF3B1-mutant MDS/MPN and SF3B1-mutant MDS (median, 57 months [95% confidence interval: 30-68] vs. 65 months [95% confidence interval: 43-85], P=0.2) patients in the Mayo Clinic cohort. (C) Kaplan-Meier estimate of overall survival between SF3B1-mutant MDS/MPN and SF3B1-mutant MDS (median, 85 months [95% confidence interval: 58-not reached] vs. 97 months [95% confidence interval: 55-118], P=0.7) patients in the Moffitt Cancer Center cohort.
haematologica | 2022; 107(5)
1191