Page 182 - Haematologica May 2022
P. 182
Letters to the Editor
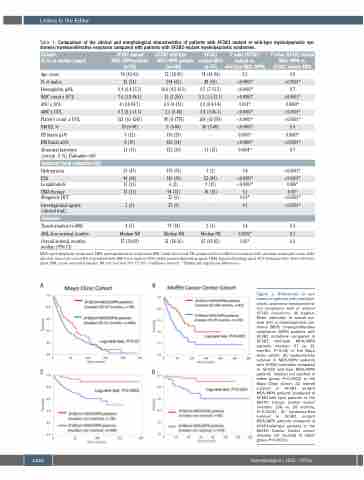
Table 1. Comparison of the clinical and morphological characteristics of patients with SF3B1 mutant or wild-type myelodysplastic syn- dromes/myeloproliferative neoplasm compared with patients with SF3B1-mutant myelodysplastic syndromes.
Variable;
N (%) or median (range)
Age, years
N. of males
Hemoglobin, g/dL
WBC count x 109/L
ANC x 109/L
AMC x 109/L
Platelet count x 109/L
BM RS, %
PB blasts ≥1%
BM blasts ≥5%
Abnormal karyotype
(except -Y, %), Evaluable=695
Treatment (total evaluable=619)
Hydroxyurea
ESA Lenalidomide
HMA therapy Allogeneic HCT
Investigational agents (clinical trial)
Outcomes
Transformation to AML AML-free survival, months
Overall survival, months; median (95% CI)
SF3B1 mutant MDS/MPN patients (n=78)
74 (43-93) 42 (54) 9.4 (6.4-13.3) 7.6 (1.8-96.1) 4 (0.4-54.7) 0.7 (0.1-11.5) 521 (63-1243) 50 (0-90)
9 (12)
8 (10)
11 (15)
33 (47)
46 (64) 15 (21)
15 (21) -
2 (3)
4 (5) Median NR 57 (30-68)
SF3B1 wild-type MDS/MPN patients
(n=446)
72 (18-95) 294 (66) 10.6 (4.2-16.9) 13 (1-265) 6.5 (0-151) 2.3 (0-40) 98 (8-1778) 0 (0-80) 130 (29) 150 (34) 123 (30)
170 (55)
120 (39) 6 (2)
SF3B1
mutant MDS (n=75)
74 (41-94) 48 (64) 9.5 (7-13.5) 5.2 (1.5-13.1) 2.9 (0.4-9.4) 0.4 (0.06-1) 268 (62-599) 40 (5-80)
-
-
11 (15)
1 (2)
52 (84) 9 (15)
P value (SF3B1 mutant vs. wild-type MDS/MPN)
0.3 <0.0001* <0.0001* <0.0001* 0.001* <0.0001* <0.0001* <0.0001* 0.0005* <0.0001* 0.0004*
0.4
<0.0001* <0.0001*
0.1 0.01*
0.1
0.4 0.0002* 0.03*
P value (SF3B1 mutant MDS/MPN vs. SF3B1 mutant MDS
0.8 <0.0001* 0.7 <0.0001* 0.0004* <0.0001* <0.0001* 0.4 0.0003* <0.0001* 0.7
<0.0001*
<0.0001* 0.006*
0.03* <0.0001*
<0.0001*
0.4 0.3 0.2
94 (32)
25(6) -
10 (16) -
2 (3) Median NR 65 (43-85)
27 (9)
79 (18) Median NR 31 (26-36)
MDS:myelodysplastic syndromes;MPN:myeloproliferative neoplasms;WBC:white blood cell;PB:peripheral blood;BM:bone marrow;ANC:absolute neutrophil count;AMC: absolute monocyte count; RS: ring sideroblasts; BM: bone marrow; ESA: erythropoiesis-stimulating agent; HMA: hypomethylating agent; HCT: hematopoietic stem cell trans- plant; AML: acute myeloid leukemia; NR: not reached; 95% CI: 95% confidence interval.. *Statistically significant differences.
Figure 1. Differences in out- comes of patients with myelodys- plastic syndrome/myeloprolifera- tive neoplasms with or without SF3B1 mutations. (A) Kaplan- Meier estimates of overall sur- vival (OS) in myelodysplastic syn- drome (MDS) /myeloproliferative neoplasms (MPN) patients with SF3B1 mutations compared to SF3B1 wild-type MDS/MPN patients (median: 57 vs. 31 months, P=0.03) in the Mayo Clinic cohort. (B) Leukemia-free survival in MDS/MPN patients with SF3B1 mutations compared to SF31B wild-type MDS/MPN patients (median not reached in either group, P=0.0002) in the Mayo Clinic cohort. (C) Overall survival in SF3B1 mutant MDS/MPN patients compared to SF3B1-wild type patients in the Moffitt Cancer Center cohort (median: 108 vs. 39 months, P<0.0001). (D) Leukemia-free survival in SF3B1 mutant MDS/MPN patients compared to SF3B1-wild-type patients in the Moffitt Cancer Center cohort (median not reached in either group, P=0.0001).
AB
CD
1190
haematologica | 2022; 107(5)