Page 178 - Haematologica May 2022
P. 178
Letters to the Editor
Table 1. Demographics and disease characteristics of the study cohort.
Characteristic
Mean age (SD)
Race/ethnicity Non-Latino-White Latino Non-Latino-Black Asian
Other
Gender Male
Female
BMI category*
Not overweight or obese Overweight or obese
ALL immunophenotype B-cell
T-cell
Minimal residual disease ^ Positive
Negative
All subjects (n=782)
7.4 (4.9)
366 (46.8) 275 (35.2) 68 (8.7) 39 (5.0) 34 (4.3)
431 (55.1) 351 (44.9)
509 (70.2) 216 (29.8)
712 (91.1) 70 (8.9)
149 (21.9)
532 (78.1)
Assigned to standard intensity induction (n=419)
4.6 (2.3)
194 (46.3) 162 (38.7) 26 (6.2) 17 (4.1) 20 (4.8)
213 (50.8) 206 (49.2)
293 (76.5) 90 (23.5)
419 (100.0) 0 (0.0)
60 (16.4)
306 (83.6)
Assigned to high intensity induction (n=363)
10.6 (5.2)
172 (47.4) 113 (31.1) 42 (11.6) 22 (6.1) 14 (3.9)
218 (60.1) 145 (39.9)
217 (63.4) 125 (36.6)
293 (80.7) 70 (19.3)
89 (28.3) 212 (71.7)
P-value <0.001
0.021
0.010 <0.001
<0.001 <0.001
*Subject numbers dependent on documented/abstracted height & weight from diagnosis. ^Subject numbers dependent on documented minimal residual disease at end
of induction. Standard intensity: standard, three-drug induction versus high intensity: four-drug Induction. SD: standard deviation; BMI: body mass index; ALL: acute lym- phoblastic leukemia.
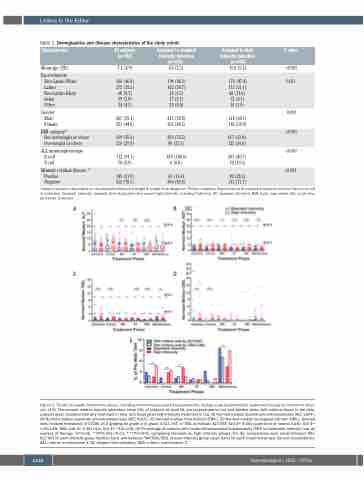
Figure 1. Trends in hepatic laboratory values, including treatment-associated hepatotoxicity, during acute lymphoblastic leukemia therapy by treatment inten- sity. (A-D) The normed median hepatic laboratory value (HL) of subjects by each HL are represented by box and whisker plots, with outliers shown in the dots, subjects given standard intensity treatment in blue, and those given high intensity treatment in red. (A) Normed median alanine aminotransaminase (ALT, SGPT). (B) Normed median aspartate aminotransaminase (AST, SGOT). (C) Normed median total bilirubin (TBIL). (D) Normed median conjugated bilirubin (CBIL). Dashed lines indicate thresholds of CTCAE v5.0 grading for grade 3 or grade 4 ALT, AST, or TBIL as follows: ALT/AST: Grd 3= 5-20x upper limit of normal (ULN), Grd 4= >20x ULN. TBIL: Grd 3= 3-10x ULN, Grd 4= >10x ULN. (E) Percentage of patients with treatment-associated hepatotoxicity (TAH) by treatment intensity over all courses of therapy. *P<0.05, **P=0.001-<0.01, ***P<0.001, comparing standard vs. high intensity groups. For (E), comparisons were made between TAH- ALT/AST of each intensity group (hashed bars) and between TAH-TBIL/CBIL of each intensity group (open bars) for each treatment phase. Consol: consolidation; IM1: interim maintenance 1; DI: delayed intensification; IM2i: interim maintenance 2.
AB
CD
E
1186
haematologica | 2022; 107(5)