Page 196 - 2022_03-Haematologica-web
P. 196
Letters to the Editor
ABC
DE
FG
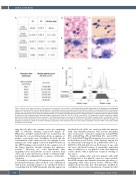
Figure 2. Blood, bone marrow features, and response to transfusion and treatment. (A) Sialotransferrin profile determined by ion-exchange chromatography using a commercial kit (CDT in Serum, Recipe München). The different isoforms were pointed out by UV detection at 460 nm and quantified by the “area percent method” (i.e., the relative abundance of each isoform is expressed as the percentage ratio of the peak area compared to the sum of the areas of all the peaks). (B) Peripheral blood smear of P2 showing enlarged platelets. (C to E) Bone marrow aspirates with an increased number of megakaryocytes at different stages of maturation. May-Grünwald-Giemsa staining; original magnification 100X (B), 10X (C), 20X (D), and 40X (E). (F) Response to platelet transfusion. Median platelet count before platelet transfusion and up to 7 days following transfusion are shown for both patients (P1 and P2’s specific values are indicated in brack- ets). (G) Time course of platelet count in response to treatments for P1 (left) and P2 (right) in response to romiplostim administration at different dosages. The dark grey bar indicates a period of complete transfusion dependency, with transfusion every 5-7 days. Values of platelets measured within 7 days after platelet transfusion are not shown.
may directly affect the enzyme active site, impairing ADP or substrate binding, respectively (Figure 3). Otherwise, p.Val516 and p.Leu517 are localized in the hydrophobic core and when mutated into an arginine or proline, respectively, may destabilize the fold and the conformation of the entire protein. Accordingly, in vitro mutagenesis of the highly conserved Asp444 residue in the ADP binding pocket resulted in the complete loss of the kinase function, though retaining the epimerase activity.13 Therefore we could hypothesize that megakaryocytes and platelets are more sensitive than other cells to defective kinetic activity or substrate-bind- ing affinity, thus explaining the occurrence of thrombo- cytopenia.
Nevertheless, whether GNE mutations are responsible for thrombocytopenia either isolated or in combination with muscle wasting remains to be elucidated. Indeed, considering that the GNE myopathy typically appears in
the third decade of life, we cannot exclude that patients with only thrombocytopenia will develop myopathy later in their life.2-4 Patients carrying the p.Asp444Tyr (F3), p.Gly447Arg (F5), p.Val516Arg (F1), p.Leu517Pro (F7), and p.Thr575Arg (F2) mutations were neonates or in their first/second decade of life. Instead, among the six individuals homozygous for p.Gly506Phe (F6) or p. Gly578Ser (F4) and all between 24-42 years of age, only two have subclinical features of myopathy, suggesting that, in addition to a low platelet count, this mutation could correlate with a mild form of muscle wasting of late onset.
Trying to explain why only few patients with GNE mutations have a low platelet count, we cannot exclude that GNE variants cause thrombocytopenia only when co- segregating with other genetic factors. Whereas WES analysis did not provide any other plausible candidate in our families, the recessive transmission of variants in other
752
haematologica | 2022; 107(3)