Page 118 - 2022_03-Haematologica-web
P. 118
S.E. Ward et al.
4A).34,35 Since sepsis-related neuraminidases may target both the N- and O-glycans of VWF, we further investigat- ed the role of MGL in clearing VWF from which both the N- and O-sialylation had been removed following diges- tion with α2-3,6,8,9 neuraminidase. In vivo clearance studies in VWF-/- mice demonstrated that combined mMGL1 and mMGL2 inhibition was not able to signifi- cantly reduce the pathological, enhanced clearance observed following loss of N-linked sialylation (Figure 6A). Interestingly, however, in mice deficient for the ASGPR clearance receptor, mMGL1/2 inhibition was associated with attenuation of the enhanced clearance of
A
α2-3,6,8,9 Neu-VWF (Figure 6B). Collectively, these find- ings further support the hypothesis that O-linked α2-3 sialylation on VWF plays a critical role in protecting against MGL-mediated clearance. Moreover, the data also suggest that loss of α2-6 sialylation (predominantly N- linked) on VWF drives enhanced clearance in a predomi- nantly MGL-independent manner, mediated through the ASGPR.
Increased VWF clearance plays a key role in the patho- genesis of both type 1 and type 2B VWD.3,11,46 Previous studies have implicated macrophages, and in particular the LRP1 and SR-A1 receptors, in regulating this enanced
BC
D
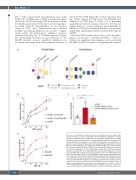
Figure 4. α2-3 sialylation on O-glycans protects von Willebrand factor against MGL- mediated clearance. (A, B) To investigate the role of terminal sialylation in modulat- ing the von Willebrand factor (VWF) interaction with MGL, plasma-derived (pd)VWF (10 mg/mL) was treated with either α2-3 neuraminidase (α2-3 Neu) to remove α2-3 linked sialylation from O-glycans, or α2-3,6,8,9 neuraminidase (α2-3,6,8,9 Neu) to remove N- and O-sialylation. Binding of the treated pdVWF sialo-glycoforms to human MGL was then compared to the binding to untreated pdVWF. (C) In order to specifi- cally focus on O-linked sialylation, pdVWF (10 mg/mL) was first digested with PNGase F to remove N-glycans and then sequentially treated with α2-3 neuraminidase (PNGase, α2-3 Neu VWF) ± β1-3 galactosidase neuraminidase (PNGase, α2-3 Neu, β1-3 Gal VWF). MGL binding was then assessed for each of the VWF O-glycan vari- ants compared to the binding of untreated pdVWF (100% binding = OD450 obtained for 10 mg/mL pdVWF). (D) To study whether other regions of VWF influence the inter- action with MGL, binding studies were compared for N-terminal D’A3-VWF compared to C-terminal A3-CK-VWF fragments. All binding experiments were performed in the presence of 1 mg/mL ristocetin. All data are shown as mean ± standard error of mean of three independent experiments. ns: not significant, *P<0.05, **P<0.01, ***P<0.001. PNGase F: peptide N-glycosidase F; OD450: absorbance at 450 nm.
674
haematologica | 2022; 107(3)