Page 142 - 2022_02-Haematologica-web
P. 142
L. Traeger et al.
subsequent transfer to the E2 enzyme, UBA6 also activates the ubiquitin-like protein FAT10, which plays a role in the immune response, obesity and aging. However, Fat10-defi- cient mice do not develop iron overload,36,37 suggesting that FAT10 does not play a direct role in iron homeostasis. In the
present study UBA6 was found to be the E1 enzyme involved in FPN regulation in vitro; depletion of UBA6, but not UBA1, prevented hepcidin-induced FPN degradation in HepG2 cells. In contrast to UBA1, which is known to charge multiple E2 enzymes with ubiquitin, UBA6 transfers
A
BC
D
EF
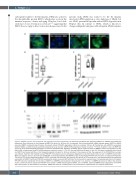
Figure 3. NDFIP1 interacts with ferroportin and regulates ferroportin degradation. (A) Cells were transfected with siControl, siNDFIP1 or siARIH1 and treated with doxycycline (Dox) (left panel) or Dox followed by BMP6 (10 ng/mL for 18 hours [h]), as indicated. Small interfering RNA (siRNA) directed against NDFIP1 or ARIH1 prevented BMP6- mediated ferroportin-green fluorescent protein (FPN-GFP) degradation. White bar indicates 100 μm. (B) Transfection with siNDFIP1 successfully depleted Ndfip1, as determined by quantitative polymerase chain reaction (qPCR) (mRNA expression relative to control; **P<0.01; Student’s t-test). (C) BMP6 (10 ng/mL for 18 h) induced hepcidin expression in siControl transfected cells. Depletion of NDFIP1 did not impair the ability of BMP6 to induce the expression of hep- cidin mRNA, as determined by qPCR (mRNA expression relative to control; **P<0.01; One-way ANOVA and Student’s t-test). (D) Cells were transfected with siControl or siNDFIP1 and treated with Dox or Dox followed by hepcidin (4 ng/mL for 18 h) as indicated. In the presence of Dox, the expression of the FPN-GFP fusion protein was induced. Treatment with hepcidin caused FPN-GFP internalization and its subsequent degradation in siControl-treated cells, but not in siNDFIP1-treated cells. White bar indicates 100 μm. (E) A low level of FPN-GFP co-immunoprecipitated with NDFIP1 in Dox-treated HepG2-FPN-GFP cells that were not treated with hepcidin. The level of FPN co-immunoprecipitating with NDFIP1 increased after treatment with hepcidin (50 ng/mL) for 20 minutes (min). Immunoprecipitation was performed using rabbit anti-NDFIP1 antibody. FPN-GFP was detected using a mouse anti-GFP antibody. The immunoblot is representative of 3 separate experiments. (F) HepG2- FPN-GFP cells were transfected with siControl-, siNDFIP1- or siNDFIP2 and incubated in the presence (+) or absence (-) of BMP6 (10 ng/mL for 18h). In the absence of Dox, control cells did not express the FPN-GFP fusion protein. (siRNA) directed against NDFIP1, but not NDFIP2, prevented BMP6-induced degradation of the FPN- GFP fusion protein, as determined by immunoblot. GAPDH was used as a loading control. The immunoblot is representative of 4 separate experiments.
484
haematologica | 2022; 107(2)