Page 316 - 2022_01-Haematologica-web
P. 316
Letters to the Editor
A
B
C
D
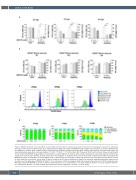
Figure 2. WNT974 treatment of primary CD34+ acute myeloid leukemia patients’ samples decreased self-renewal but not apoptosis or quiescence. (A) Colony- forming unit (CFU) assay of CD34+ primary acute myeloid leukemia (AML) cells treated with WNT974 drug at different concentrations or vehicle (dimethylsulfox- ide, DMSO 0.1% volume). After 2 weeks, colonies were scored and replated. A second scoring was done 2 weeks after replating. The graph shows mean and standard deviation values from a technical triplicate. *P<0.05, **P<0.01, ***P<0.001 (t-test). (B) CFU assay of CD34+ cells from bone marrow of adult healthy donors treated with WNT974 drug at different concentrations or vehicle (DMSO 0.1% volume). After 2 weeks, colonies were scored and replated. A second scor- ing was done 2 weeks after replating. All tests (t-tests) were non-significant. (C) CellTrace Violet assay of three CD34+ selected primary AML cells treated with WNT974 at various concentrations, or vehicle (DMSO 0.1%). 7-AAD staining was conducted to select only the living cells. The graphs show the different counts at day 0 (dark blue, only control), and at day 6 (3 days of incubation, plus 3 days of treatment or vehicle). No values are statistically significant except for that for patient 2 at the higher dose of WNT974 (P=0.0077, t-test). (D) Annexin V-FITC and propidium iodide (PI) analysis by flow cytometry of CD34+ selected primary AML cells treated with WNT974 or control at different concentrations for 72 h. Living (Annexin–, PI–), Early apoptosis (Annexin+, PI–), Late apoptosis (Annexin+, PI+) and Necrosis (Annexin–, PI+). Each analysis was conducted in technical triplicates. *P<0.05, **P<0.01 (t-test).
308
haematologica | 2022; 107(1)