Page 43 - 2021_12-Haematologica-web
P. 43
Frequent RUNX1 mutations in acute leukemia + pDC
and even than in normal pDC (MFIR: 16.9 [range, 3.5- 45.5], P=0.0052; MFI: 8,106 [range, 3,389-3,1544], P=0.0192) (Figure 3C and D).
Interestingly, four patients with M0-AML expressed myeloid, B-cell or T-cell markers on both CD34+ blasts and pDC, but with partial and lower expression on pDC than on CD34+ blasts: CD33 and CD22 for N7, CD13 for N1, CD33 for N36, CD5 and CD7 for N20 (Figure 2). A maturation continuum between immature blasts and pDC was observed in some cases (Figure 1D).
cDC were identified by the expression of CD1c and CD11c without CD14 and CD64 expression in patients N7 and N8, and were sorted for molecular studies for patient N8 only.
Mutational profile
The purity of each sorted fraction is depicted in Online Supplementary Table S3. The NGS panel was informative for all cases, with a number of detected mutations ranging from two to six. Fourteen genes were found to be mutated among the 70 explored (Figure 4), corresponding to tran- scription factors RUNX1 (11 of 15, 73%); epigenetic mod- ifiers ASXL1 (five of 15, 33%), EZH2 (three of 15, 20%), TET2 (four of 15, 27%), DNMT3A (three of 15, 20%); genes involved in splicing SRSF2 (five of 15, 33%), SF3B1 (two of 15, 13%), U2AF1 (one of 15); RAS pathway CBL (three of 15, 19%), KRAS, PTPN11 (one of 15 each); cytokine signaling FLT3 (three of 15, 19%) as well as other genes, such as PFH6 and WT1 (one of 15 each) (Online Supplementary Table S4). Of note, only one case was mutated for NPM1 (patient N35) and none for CEBPA. In
A
BPDCN, 17 out of the 21 of the cohort were studied by NGS, with zero to five mutations per case on 17 genes: TET2 (nine of 17, 53%), ASXL1 (six of 17, 41%), ZRSR2 (four of 17, 24%), TP53 (three of 17, 18%), IKZF1, NRAS, SRSF2, IDH1 (two of 17 each, 12%), ZEB2, MET, ETV6, ATM, IKZF3, CXCR4, NOTCH2, KRAS, JAK2 (one of 17 each) (Figure 4).
Mutations were systematically found in sorted CD34+ immature blasts, pDC, monocyte and cDC of the same sample, and were not detected in the T-cell fraction, thus confirmed to be a non-neoplastic subpopulation (Figure 4). Variant allele frequencies (VAF) were quite similar between cell fractions. However, VAF of the monocyte subpopulation were lower than in blasts and pDC in two cases (N13 and N36), which may indicate that this muta- tion is subclonal in monocytes (N13) or that there is a mix- ture of neoplastic and reactive non-neoplastic monocytes (same VAF difference in all detected mutations for N36). Some mutations were subclonal in both blast and mono- cyte fractions: KRAS for N19, FLT3 for N20 (Figure 4). Overall, the most frequently mutated genes were the tran- scription factor RUNX1, splicing genes (SRSF2, SF3B1, U2AF1) and epigenetic modifiers DNMT3A and TET2. Interestingly, RUNX1 mutations concerned all M0-AML, while none of the other cases were mutated (M4/5-AML and M1-AML). Consequently, despite the low number of cases, there was a significant association between the M0- AML subtype and RUNX1 mutations (c2 with Yates' cor- rection, X2=10.32, 1df, P=0.0013). The majority of RUNX1 mutations detected were frameshift (n=6) or stop gain (n=3), with a biallelic invalidation in patient N13. RUNX1-
B
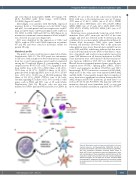
Figure 5. Maturation model in plasmacytoid dendritic cell-acute myeloid leukemia. (A) Representative CD45/SSC dot plot of plasmacytoid dendritic cells-acute myeloid leukemia (pDC-AML), with four populations identified: immature CD34+ blasts in black, pDC in pink, monocytes in green and lymphocyte in blue, with morphologies of these populations depicted above. (B) The maturation model: immature blast cells are mainly proliferative without maturation, but at least part of them conserved MDP (macrophage-DC progenitor)-like potential of maturation leading to variable amounts of clonal pDC, monocytes and conventional DC (cDC).
haematologica | 2021; 106(12)
3063