Page 126 - 2021_12-Haematologica-web
P. 126
S. Bohler et al.
common reason for treatment delays or dose reductions. We, therefore, consider it important to generate preclini- cal data to evaluate the hematotoxicity profile of such novel drugs. Recently, we described the detrimental effects of BCL-XL inhibition on human HSPC and ery- throid progenitors.31
Here, we dissected the effects of MCL-1 inhibition on different immature and mature types of hematologic cells. By using an RNA interference approach and the spe- cific small molecule inhibitor S63845, we consistently found that MCL-1 expression is crucial for multipotent stem and progenitor cells, as well as for myeloid progen- itors, while erythroid progenitors are less susceptible to MCL-1 inhibition. During later stages of blood cell differ- entiation, MCL-1 becomes dispensable for cell survival. Interestingly, we noted re-expression of MCL-1 mRNA after some days of culture, when shRNA #4 was used, while the shRNA #3 resulted in stable knockdown. Nevertheless, the resulting phenotypes were strikingly similar indicating that the loss of stem and multipotent progenitors occurs early after MCL-1 depletion and can- not be compensated by later MCL-1 re-expression.
Looking more closely at the stem and progenitor cell compartment, we noted that cells that specifically enter
the differentiation process are highly dependent on MCL- 1 expression, while proliferating CD34+ cells remain fairly resistant. What is the reason for this difference? It is pos- sible that the differentiation process is associated with increased stress levels reflected by accumulation of acti- vated BH3-only proteins. Alternatively, it is possible that the cytokines TPO, FLT3L, SCF and IL3, which induce cell proliferation and are used for CD34+ cell culture, not only induce proliferation but also confer CD34+ cells with sur- vival signals, thereby rendering them independent of MCL-1 expression. Indeed, we have shown earlier that these cytokines induce BCL-XL mRNA upregulation and at the same time repress expression of the pro-apoptotic BCL-2 proteins BIM and BMF.30 Also in this study, BIM mRNA levels were lower and BCL-XL mRNA levels high- er when cells were cultured in the presence of TPO, FLT3L, SCF and IL-3. Delbridge et al. recently showed that MCL-1 expression in murine HSPC is critically required to counteract PUMA-induced apoptosis. While mice lacking only one Mcl-1 allele in the hematopoietic system rapidly succumbed to bone marrow failure, addi- tional deletion of both Puma alleles rescued all animals.26 However, our in vitro studies showed strong upregulation of PUMA mRNA in human CD34+ cells irrespective of the
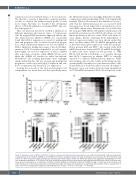
AB
CD
Figure 8. Synergistic action of MCL-1 and BCL-XL inhibitors on human hematopoietic stem and progenitor cells. (A-B) Cord blood CD34+ cells were treated with increasing doses of the inhibitors S63845 and A-1155463 to determine synergism between the drugs. (A) Apoptosis was measured 24 h after treatment using annexin V/7-AAD. The percent specific apoptosis was calculated. (B) A dose-response matrix was determined using the web application SynergyFinder (n=4 from four independent experiments). (C-D) Human CD34+ cells, derived from either cord blood or bone marrow, were differentiated in MethoCult culture (1,000 cells seed- ed per plate) in the presence of a combination of the MCL-1 inhibitor S63845 and the BCL-XL inhibitor A-1155463 (0.1 mM or 1 mM of each). After 10 days, total colony numbers (C) and total cell numbers (D) were determined by light microscopy and hemocytometry, respectively. Bars represent mean ± standard error of mean; n=6-7 from six independent experiments. Mann-Whitney test: *P<0.05, **P<0.01 ***P<0.001, ****P<0.0001.
3146
haematologica | 2021; 106(12)