Page 237 - 2021_10-Haematologica-web
P. 237
Letters to the Editor
AB
C
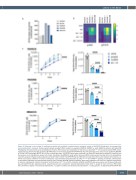
Figure 2. Enhanced in vivo activity of combined ponatinib and ruxolitinib in patient-derived xenograft models of IGH-EPOR Philadelphia chromosome-like acute lymphoblastic leukemia. Tertiary patient-derived xenograft (PDX) models from pediatric (PAVRCK, PAVDRS) or adult (MDACC3) patients with IGH-EPOR Philadelphia chromosome-like acute lymphoblastic leukemia (Ph-like ALL) were established in NSG mice as described.5,9,11 (A) Increased surface EPOR protein expression was detected by flow cytometry (BD FACSVerse) in two of three tested IGH-EPOR Ph-like ALL PDX specimens (blue bars) versus non-Ph-like NALM-6 or Ph-like CRLF2-rearranged MUTZ5 ALL cell line controls (grey bars). IGH-EPOR fusions were confirmed in all three PDX specimens by ArcherDX FusionPlex and fluorescence in situ hybridization assays (not shown), including in the MDACC3 model without increased EPOR surface protein staining. (B) In vitro phosphoflow cytometry analysis of thawed viably-cryopreserved CD10+/CD19+/CD45+ IGH-EPOR Ph-like ALL PDX cells harvested from NSG murine spleens demonstrates rux- olitinib (rux)-induced inhibition of human erythropoietin (epo)-stimulated phosphorylated (p) JAK2Y1007/1008 and STAT5Y694 signaling. Conversely, erythropoietin co-stimulation effectively rescued potential ponatinib (pon)-induced JAK/STAT signaling inhibition. Both kinase inhibitors were used at 1 mM with simultaneous 5 units/mL erythropoietin cytokine stimulation for 60 minutes at 37°C in a 5% CO2 incubator. NALM-6 and MUTZ5 cells were used as non-Ph-like and JAK path- way-activated Ph-like ALL signaling controls, respectively. Data are normalized to basal phosphoprotein levels (level =1, purple) for each cell line or PDX model with colorimetric display of increased phosphorylation >1 (green-to-yellow) and decreased phosphorylation <1 (deep purple). (C) Engrafted IGH-EPOR Ph-like
haematologica | 2021; 106(10)
2765