Page 102 - 2021_10-Haematologica-web
P. 102
B. Maurer et al.
while other blood parameters and erythroid development are unaltered, which may be explained by a slightly reduced cell cycle progression in reticulocytes in the absence of CDK4. Alterations in erythroid development are most likely linked to the additional functions of CDK6 beyond its role as a cell cycle kinase but as tran- scriptional regulator and contributor to the cytoskeleton of erythroid cells.6
CDK6 plays a minor role under homeostatic conditions for HSC maintenance in complete Cdk6-/- mice where the most dormant HSC start to accumulate.20 Upon deletion of CDK6 during adult hematopoiesis the numbers of all HSC fractions are elevated, which is not observed in the BM of Cdk4D/D mice. The global expansion of the stem/progenitor pool in Cdk6D/D compared to Cdk6-/- may reflect a key role for CDK6 at all HSC stages which is compensated for in the total knockout. The poly(I:C)-trig- gered interferon storm may also contribute to the differ- ences as interferons induce HSC cycling.41,42 However, the time frames chosen make poly(I:C) effects unlikely as associated changes like increased Sca-1 expression resolve within 8 days.43 CDK6, but not CDK4, has been implicat- ed in stress-induced hematopoiesis. Murine Cdk6-/- cells only survive oncogenic stress by mutating the tumor sup- pressor Tp53 – a mechanism that has been confirmed in leukemia patients.17 The Cdk6fl/fl model now opens the
opportunity of studying the consequences of CDK6 dele- tion in already established cancers circumventing Tp53 mutations.
Cdk4-/- mice have not been reported to exert hematopoietic abnormalities.11,14 We found that CDK4 deletion in the murine adult hematopoietic system first triggers the accumulation of myeloid progenitors in the BM presumably contributing to the observed increase in BM cellularity, which declines at a later time point. These differences do not translate into different numbers of CD11b+Gr1hi granulocytes in Cdk4D/D mice. Conversely, a significant reduction of CD11b+Gr1hi granulocytes in Cdk6D/D mice is seen, which probably induces a compen- satory expansion of myeloid progenitors as observed 6 weeks after Cdk6 deletion. The increase in erythroid pro- genitors after 6 weeks supports the concept of a compen- sation taking place as response to the decreased numbers of mature erythroid cells. As aberrant type I interferon signaling accelerates myelopoiesis,44 the poly(I:C)- induced interferon signaling might contribute to the ini- tial abnormal expansion of myeloid progenitors in Cdk4D/D mice, while differentiation and maturation stay unal- tered. During myeloid differentiation interleukin (IL)-6, IL-1 and IL-17 act on MPP and promote the production of myeloid cells in a feed-forward loop.45,46 CDK6 regulates cytokine expression in hematopoiesis18 and thus may
AB
CD
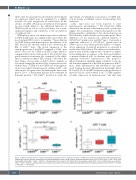
Figure 4. CDK4 and CDK6 drive the myeloid and lymphoid progenitor pool in opposite directions. Analysis was performed 3 weeks post final polyinosinic–poly- cytidylic acid (poly(I:C)) injection. Percentage of (A) myeloid progenitors (LKS-) and (B) common lymphoid progenitors (CLP) (Lin-IL-7R+c-KitmidSca-1mid) per 2.5x106 bone marrow (BM) cells (Cdk4∆/∆; Cdk6∆/∆; Cdk4/6fl/fl, n≥5/genotype). Total numbers of (C) CD11b+Gr1hi granulocytes and (D) CD3e+ T cells in BM (two femurs and tibias; Cdk4∆/∆; Cdk6∆/∆; Cdk4/6fl/fl, n≥8/genotype). All comparisons were done with one-way ANOVA followed by Bonferroni’s Multiple Comparison Test, *P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001.
2630
haematologica | 2021; 106(10)