Page 260 - 2021_06-Haematologica-web
P. 260
2288
Case Reports
A
B
C
D
E
F
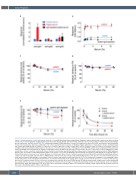
Figure 2. Characterization of anti-emicizumab antibodies. (A and B) Emicizumab was immobilized (5 mg/mL) and incubated with control serum (blue), patient serum (red) or immunoglobulin G (IgG)-depleted patient serum (black). Bound anti-emicizumab antibodies were probed using peroxidase-labeled IgG-subtype specific antibodies, and detected via 3,3’,5,5’-tetramethyl benzidine (TMB) hydrolysis. For panel A, samples were diluted 256-fold, and response was normalized to that of normal plasma, which was arbitrarily set at 1. For panel B, the dose-response for binding of IgG1 antibodies to emicizumab is shown. (C) Emicizumab (25 mg/mL) was incubated in the absence or presence of various dilutions of control serum (blue circles) or patient serum (red circles). Presented is the per- centage residual emicizumab activity relative to the absence of serum as measured in a chromogenic factor VIII (FVIII)-activity assay using human FIXa and factor X (FX). (D) Binding of bt-emicizumab (50 mg/mL) to immobilized factor IX (FIX) (5 mg/mL) was performed in the absence or presence of various dilutions of control serum (blue circles) or patient serum (red circles). Bound bt-emicizumab was probed with peroxidase-labeled streptavidin and detected via TMB hydrolysis. Shown is the percentage residual FIX binding relative to the absence of serum. (E) Binding of bt-emicizumab (10 mg/mL) to immobilized FX (5 mg/mL) was per- formed in the absence or presence of various dilutions of control serum (blue circles) or patient serum (red circles). Bound bt-emicizumab was probed with per- oxidase-labeled streptavidin and detected via TMB hydrolysis. Shown is the percentage residual FX binding relative to the absence of serum. Statistical assess- ment was performed using multiple t-test analysis between control and patient serum. Stars indicate P<0.05 as analyzed in a multiple t-test comparing control serum and patient serum. (F) Immuno-deficient mice received bt-emicizumab (0.25 mg/kg) alone (orange circles) or in the presence of control serum (100 mL; blue circles) or patient serum (100 mL; red circles) via intravenous injection in the retro-orbital plexus. At indicated time points, samples were taken and plasma was analyzed for the presence of residual bt-emicizumab. Presented is the percentage of residual bt-emicizumab relative to bt-emicizumab alone at 3 minutes after injection, which was arbitrarily set at 100%. Lines were generated by fitting the data to an equation describing a single-exponential decay. Data represent the mean ± standard deviation of three experiments.
haematologica | 2021; 106(8)