Page 19 - 2021_06-Haematologica-web
P. 19
The post-HCT microbiome: effects and targeted therapy
A
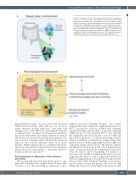
Figure 2. Interactions between the gut microbiota and mucosal immune system in steady state and peri-transplant environments. (A) Pre-trans- plant interaction between the intestinal tract, mucosal immune system and the microbiome. (B) Peri-transplant interaction between the inflamed intestinal tract, infiltrating donor populations, some of which alloreactive T cells that cause graft-versus-host disease, and the perturbed microbiome, often colonized with potential pathogens, such as Enterococcus faecalis/faecium. APC: antigen-presenting cell; DAMPS: damage-associat- ed patterns; GvHD: graft-versus-host disease; LPS: lipopolysaccharides; PAMPS: pathogen-associated patterns. Created with BioRender.com
B
piperacillin/tazobactam, was associated with increased GvHD-related mortality while this was not observed when anaerobic-sparing antibiotics, aztreonam and cefepime, were used.32 Of note, shortening the duration of administration of empiric broad-spectrum antibiotics in patients with febrile neutropenia without an identifi- able infectious cause has been studied and appears to be safe; however, it remains unclear whether the shortened exposure also reduced intestinal dysbiosis in these patients.83 Other strategies that are currently being devel- oped to prevent antibiotic-mediated microbial disruption include molecules that degrade or neutralize antibiotic residues in the intestinal tract.84,85
Pre-transplantation optimization of the intestinal microbiome
Since a relatively preserved microbiome prior to allo- geneic HCT translates into a higher chance of a favorable outcome,30 it is worth considering a potential role for pre-
emptive microbiota-targeting therapies. Two studies examined the effect of prebiotic supplementation prior to HCT: a prospective study by Yoshifuji and colleagues demonstrated that adequate intake of resistant starch and GFO from day -7 before allogeneic HCT to day +28 after the transplant reduced the incidence of acute GvHD and preserved populations of butyrate-producing bacteria, while fecal butyrate concentration in samples after trans- plantation did not differ significantly between GFO-treat- ed patients and historical controls.86 The patients with ini- tial high microbial diversity in this cohort appeared more likely to benefit from prebiotic supplementation (n=14 of 30 in the whole cohort). Remarkably, the incidence of late-onset acute GvHD was higher in the prebiotic group, suggesting that there is only a temporary benefit from prebiotic supplementation and that the benefit subsides after cessation of the treatment. Similarly, a second retro- spective study observed that patients who received GFO during the same period had higher survival rates early
haematologica | 2021; 106(8)
2047